Etazolate
Appearance
(Redirected from C14H19N5O2)
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
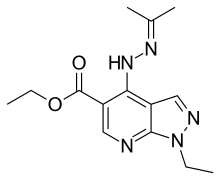
| Formula | C14H19N5O2 |
| Molar mass | 289.339 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties.[1][2][3] It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site,[4][5][6][7] as an adenosine antagonist of the A1 and A2 subtypes,[8] and as a phosphodiesterase inhibitor selective for the PDE4 isoform.[9][10][11] It is currently in clinical trials for the treatment of Alzheimer's disease.[12]
See also
[edit]References
[edit]- ^ Hall JA, Morton I (1999). Concise dictionary of pharmacological agents: properties and synonyms. Kluwer Academic. ISBN 0-7514-0499-3.
- ^ Williams M (May 1983). "Anxioselective anxiolytics". Journal of Medicinal Chemistry. 26 (5): 619–628. doi:10.1021/jm00359a001. PMID 6132997.
- ^ Williams M, Risley EA (February 1979). "Enhancement of the binding of 3H-diazepam to rat brain membranes in vitro by SQ 20009, A novel anxiolytic, gamma-aminobutyric acid (GABA) and muscimol". Life Sciences. 24 (9): 833–841. doi:10.1016/0024-3205(79)90367-9. PMID 449623.
- ^ Zezula J, Slany A, Sieghart W (April 1996). "Interaction of allosteric ligands with GABAA receptors containing one, two, or three different subunits". European Journal of Pharmacology. 301 (1–3): 207–214. doi:10.1016/0014-2999(96)00066-0. PMID 8773466.
- ^ Davies MF (1996). "The Pharmacology of the Gamma-Aminobutyric Acid System". In Remington G, Baskys A (eds.). Brain mechanisms and psychotropic drugs. Boca Raton: CRC Press. ISBN 0-8493-8386-2.
- ^ Olsen RW, Gordey M (2000). "GABAA Receptor Chloride Ion Channels". In Mishina M, Kurachi Y (eds.). Pharmacology of ionic channel function: activators and inhibitors. Handbook of Experimental Pharmacology. Vol. 147. Berlin: Springer. pp. 499–517. doi:10.1007/978-3-642-57083-4_19. ISBN 3-540-66127-1.
- ^ Olsen RW (1987). "GABA-Drug Interactions". In Jucker E (ed.). Progress in Drug Research. Vol. 31. Boston: Birkhauser. p. 526. ISBN 3-7643-1837-6.
- ^ Williams M, Jarvis MF (February 1988). "Adenosine antagonists as potential therapeutic agents". Pharmacology, Biochemistry, and Behavior. 29 (2): 433–441. doi:10.1016/0091-3057(88)90182-7. PMID 3283781. S2CID 35635747.
- ^ Chasin M, Harris DN, Phillips MB, Hess SM (September 1972). "1-Ethyl-4-(isopropylidenehydrazino)-1H-pyrazolo-(3,4-b)-pyridine-5-carboxylic acid, ethyl ester, hydrochloride (SQ 20009)--a potent new inhibitor of cyclic 3',5'-nucleotide phosphodiesterases". Biochemical Pharmacology. 21 (18): 2443–2450. doi:10.1016/0006-2952(72)90414-5. PMID 4345859.
- ^ Wang P, Myers JG, Wu P, Cheewatrakoolpong B, Egan RW, Billah MM (May 1997). "Expression, purification, and characterization of human cAMP-specific phosphodiesterase (PDE4) subtypes A, B, C, and D". Biochemical and Biophysical Research Communications. 234 (2): 320–324. doi:10.1006/bbrc.1997.6636. PMID 9177268.
- ^ Daniel JL (2002). "Platelet signalling; cAMP and cGMP". In Gresele P (ed.). Platelets in thrombotic and non-thrombotic disorders: pathophysiology, pharmacology and therapeutics. Cambridge, UK: Cambridge University Press. ISBN 0-521-80261-X.
- ^ "EHT 0202". Pipeline. ExonHit. Archived from the original on 2011-01-11.
