Substituted cathinone


Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions.[1][2][3][4][5] Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.[6]
Substituted cathinones act as monoamine releasing agents and/or monoamine reuptake inhibitors, including of norepinephrine, dopamine, and/or serotonin.[7][8][9][10][11][12] In contrast to substituted amphetamines, substituted cathinones do not act as agonists of the human trace amine-associated receptor 1 (TAAR1).[13][14][15] This may potentiate their stimulating and addictive effects.[13][14] In addition, β-keto-substituted phenethylamines, such as βk-2C-B, appear to show dramatically reduced potency and efficacy as serotonin 5-HT2A receptor agonists compared to their non-β-keto-substituted counterparts.[16]
List of substituted cathinones
[edit]The derivatives may be produced by substitutions at four locations of the cathinone molecule:
- R1 = hydrogen, or any combination of one or more alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents
- R2 = hydrogen or any alkyl group
- R3 = hydrogen, any alkyl group, or incorporation in a cyclic structure
- R4 = hydrogen, any alkyl group, or incorporation in a cyclic structure
The following table displays notable derivatives that have been reported:[17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38]
| Structure | Compound | R1 | R2 | R3 | R4 | CAS number |
|---|---|---|---|---|---|---|
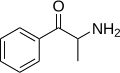 |
Cathinone | H | Me | H | H | 71031-15-7 |
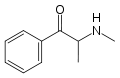 |
Methcathinone | H | Me | H | Me | 5650-44-2 |
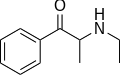 |
Ethcathinone | H | Me | H | Et | 51553-17-4 |
 |
Propylcathinone | H | Me | H | nPr | 52597-14-5 |
 |
Buphedrone | H | Et | H | Me | 408332-79-6 |
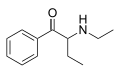 |
N-Ethylbuphedrone (NEB) | H | Et | H | Et | 1354631-28-9 |
 |
N-Methyl-N-ethylbuphedrone | H | Et | Me | Et | |
 |
Pentedrone | H | nPr | H | Me | 879722-57-3 |
 |
N-Ethylpentedrone | H | nPr | H | Et | 18268-16-1 |
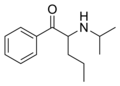 |
N-Isopropylpentedrone | H | nPr | H | iPr | 18268-14-9 |
 |
Hexedrone | H | nBu | H | Me | 2169446-41-5 |
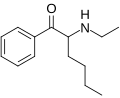 |
N-Ethylhexedrone | H | nBu | H | Et | 18410-62-3 |
 |
N-Butylhexedrone | H | nBu | H | nBu | 18296-66-7 |
 |
N-Isobutylhexedrone (NDH) | H | nBu | H | i-Bu | |
 |
Isohexedrone | H | iBu | H | Me | |
 |
N-Ethylheptedrone | H | nPe | H | Et | |
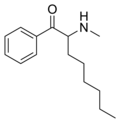 |
Octedrone | H | hexyl | H | Me | |
 |
Dimethylcathinone | H | Me | Me | Me | 15351-09-4 |
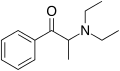 |
Diethylpropion | H | Me | Et | Et | 134-80-5 |
 |
N-Methyl-N-ethylcathinone | H | Me | Me | Et | 1157739-24-6 |
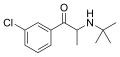 |
Bupropion | 3-Cl | Me | H | t-Bu | 34911-55-2 |
 |
Hydroxybupropion | 3-Cl | Me | H | 2-Me-3-OH-propan-2-yl | 357399-43-0 |
 |
Mephedrone | 4-Me | Me | H | Me | 1189805-46-6 |
 |
2-MMC | 2-Me | Me | H | Me | 1246911-71-6 |
 |
2-MEC | 2-Me | Me | H | Et | 1439439-84-5 |
 |
2-EMC | 2-Et | Me | H | Me | |
 |
2-EEC | 2-Et | Me | H | Et | 2446466-59-5 |
 |
3-MMC | 3-Me | Me | H | Me | 1246816-62-5 |
 |
3-MEC | 3-Me | Me | H | Et | 1439439-83-4 |
 |
3-MPC | 3-Me | Me | H | nPr | |
 |
3-EMC | 3-Et | Me | H | Me | |
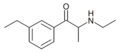 |
3-EEC | 3-Et | Me | H | Et | 2446466-61-9 |
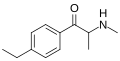 |
4-EMC | 4-Et | Me | H | Me | 1225622-14-9 |
 |
4-EEC | 4-Et | Me | H | Et | 2446466-62-0 |
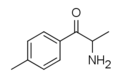 |
4-MC | 4-Me | Me | H | H | 31952-47-3 |
 |
Benzedrone | 4-Me | Me | H | Bn | 1225617-75-3 |
 |
2'-MeO-Benzedrone | 4-Me | Me | H | 2-MeO-Bn | |
 |
2,N-Dimethylbenzedrone | 2-Me | Me | Me | Bn | |
 |
3,N-Dimethylbenzedrone | 3-Me | Me | Me | Bn | |
 |
4,N-Dimethylbenzedrone | 4-Me | Me | Me | Bn | |
 |
4-MEC | 4-Me | Me | H | Et | 1225617-18-4 |
 |
4-MPC | 4-Me | Me | H | nPr | |
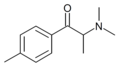 |
N,N-DMMC | 4-Me | Me | Me | Me | 1448845-14-4 |
 |
N,N-MEMC | 4-Me | Me | Me | Et | |
 |
N,N-DEMC | 4-Me | Me | Et | Et | 676316-90-8 |
 |
4-MEAP | 4-Me | Pr | H | Et | 746540-82-9 |
 |
EDMC | 4-Et | Me | Me | Me | |
 |
2,3-DMMC | 2,3-dimethyl | Me | H | Me | |
 |
2,3-DMEC | 2,3-dimethyl | Me | H | Et | |
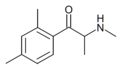 |
2,4-DMMC | 2,4-dimethyl | Me | H | Me | 1225623-63-1 |
 |
2,4-DMEC | 2,4-dimethyl | Me | H | Et | 1225913-88-1 |
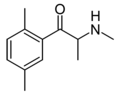 |
2,5-DMMC | 2,5-dimethyl | Me | H | Me | |
 |
2,5-DMEC | 2,5-dimethyl | Me | H | Et | |
 |
2,6-DMMC | 2,6-dimethyl | Me | H | Me | |
 |
2,6-DMEC | 2,6-dimethyl | Me | H | Et | |
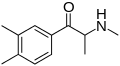 |
3,4-DMMC | 3,4-dimethyl | Me | H | Me | 1082110-00-6 |
 |
3,4-DMEC | 3,4-dimethyl | Me | H | Et | 1225811-81-3 |
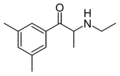 |
3,5-DMEC | 3,5-dimethyl | Me | H | Et | |
 |
2,4,5-TMMC | 2,4,5-trimethyl | Me | H | Me | 1368603-85-3 |
 |
2,4,5-TMOMC | 2,4,5-trimethoxy | Me | H | Me | |
 |
3,4,5-TMOMC | 3,4,5-trimethoxy | Me | H | Me | |
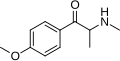 |
Methedrone | 4-MeO | Me | H | Me | 530-54-1 |
 |
Dimethedrone | 4-MeO | Me | Me | Me | 91564-39-5 |
 |
Ethedrone | 4-MeO | Me | H | Et | |
 |
2-MOMC | 2-MeO | Me | H | Me | |
 |
3-MOMC | 3-MeO | Me | H | Me | 1435933-70-2 |
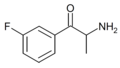 |
3-FC | 3-F | Me | H | H | 1082949-91-4 |
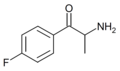 |
4-FC | 4-F | Me | H | H | 80096-51-1 |
 |
2-FMC | 2-F | Me | H | Me | 1186137-35-8 |
 |
2-FEC | 2-F | Me | H | Et | |
 |
3-FMC | 3-F | Me | H | Me | 1049677-77-1 |
 |
3-FEC | 3-F | Me | H | Et | |
 |
2-CMC | 2-Cl | Me | H | Me | |
 |
2-BMC | 2-Br | Me | H | Me | |
 |
2-IMC | 2-I | Me | H | Me | |
 |
2-TFMAP | 2-CF3 | Me | H | Me | |
 |
Clophedrone (3-CMC) | 3-Cl | Me | H | Me | 1049677-59-9 |
 |
3-CEC | 3-Cl | Me | H | Et | 2150476-60-9 |
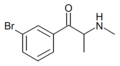 |
3-BMC | 3-Br | Me | H | Me | 676487-42-6 |
 |
3-IMC | 3-I | Me | H | Me | |
 |
3-TFMAP | 3-CF3 | Me | H | Me | |
 |
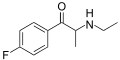
Flephedrone | 4-F | Me | H | Me | 447-40-5 |
 |
4-FEC | 4-F | Me | H | Et | 1225625-74-0 |
 |
Clephedrone (4-CMC) | 4-Cl | Me | H | Me | 1225843-86-6 |
 |
2-CEC | 2-Cl | Me | H | Et | |
 |
4-CEC | 4-Cl | Me | H | Et | 14919-85-8 |
 |
2-CiPC | 2-Cl | Me | H | iPr | |
 |
3-CiPC | 3-Cl | Me | H | iPr | |
 |
4-CiPC | 4-Cl | Me | H | iPr | |
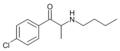 |
4-CBC | 4-Cl | Me | H | nBu | 1225621-71-5 |
 |
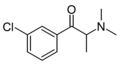
2-CDMC | 2-Cl | Me | Me | Me | |
 |
3-CDMC | 3-Cl | Me | Me | Me | |
 |
4-CDMC | 4-Cl | Me | Me | Me | 1157667-29-2 |
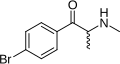 |
Brephedrone | 4-Br | Me | H | Me | 486459-03-4 |
 |
4-BEC | 4-Br | Me | H | Et | 135333-26-5 |
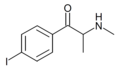 |
4-IMC | 4-I | Me | H | Me | |
 |
4-TFMAP | 4-CF3 | Me | H | Me | |
 |
4-EFMC | 4-(2-fluoroethyl) | Me | H | Me | |
 |
4-MTMC | 4-SCH3 | Me | H | Me | |
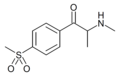 |
4-MSMC | 4-SO2CH3 | Me | H | Me | |
 |
4-PHMC | 4-phenyl | Me | H | Me | |
 |
Mexedrone | 4-Me | methoxymethyl | H | Me | |
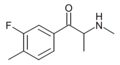 |
FMMC | 3-F-4-Me | Me | H | Me | 1696642-00-8 |
 |
MFMC | 3-Me-4-F | Me | H | Me | 1368943-21-8 |
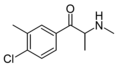 |
4-Cl-3-MMC | 3-Me-4-Cl | Me | H | Me | |
 |
MMOMC | 3-Me-4-MeO | Me | H | Me | |
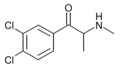 |
3,4-DCMC | 3,4-dichloro | Me | H | Me | 802281-39-6 |
 |
3,4-DCEC | 3,4-dichloro | Me | H | Et | 1225618-63-2 |
 |
3,5-DCMC | 3,5-dichloro | Me | H | Me | |
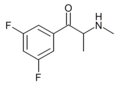 |
3,5-DFMC | 3,5-difluoro | Me | H | Me | 1430343-55-7 |
 |
2,5-DMOMC | 2,5-dimethoxy | Me | H | Me | |
 |
βk-2C-C | 2,5-dimethoxy-4-chloro | H | H | H | 1538191-15-9 |
 |
βk-2C-B | 2,5-dimethoxy-4-bromo | H | H | H | 807631-09-0 |
 |
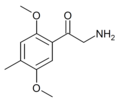
βk-2C-I | 2,5-dimethoxy-4-iodo | H | H | H | |
 |
βk-2C-D | 2,5-dimethoxy-4-methyl | H | H | H | 1368627-25-1 |
 |
βk-2C-E | 2,5-dimethoxy-4-ethyl | H | H | H | 1517021-02-1 |
 |
βk-2C-P | 2,5-dimethoxy-4-propyl | H | H | H | |
 |
βk-2C-iP | 2,5-dimethoxy-4-isopropyl | H | H | H | 1511033-62-7 |
 |
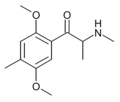
βk-DOB | 2,5-dimethoxy-4-bromo | Me | H | H | |
 |
βk-MDOM | 2,5-dimethoxy-4-methyl | Me | H | Me | |
 |
βk-MDA | 3,4-methylenedioxy | Me | H | H | 80535-73-5 |
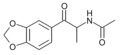 |
N-Acetyl-βk-MDA | 3,4-methylenedioxy | Me | H | acetyl | |
 |
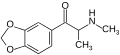
2,3-MDMC | 2,3-methylenedioxy | Me | H | Me | 1427205-87-5 |
 |
Methylone | 3,4-methylenedioxy | Me | H | Me | 186028-79-5 |
 |
Dimethylone | 3,4-methylenedioxy | Me | Me | Me | 109367-07-9 |
 |
N-Acetylmethylone | 3,4-methylenedioxy | Me | acetyl | Me | |
 |
N-Hydroxymethylone | 3,4-methylenedioxy | Me | hydroxy | Me | |
 |
Ethylone | 3,4-methylenedioxy | Me | H | Et | 1112937-64-0 |
 |
Diethylone | 3,4-methylenedioxy | Me | Et | Et | |
 |
N-Acetylethylone | 3,4-methylenedioxy | Me | acetyl | Et | |
 |
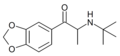
N-Isopropyl-βk-MDA | 3,4-methylenedioxy | Me | H | iPr | |
 |
MDPT | 3,4-methylenedioxy | Me | H | t-Bu | 186028-84-2 |
 |
Benzylone (BMDP) | 3,4-methylenedioxy | Me | H | Bn | 1823274-68-5 |
 |
N-Cyclohexylmethylone | 3,4-methylenedioxy | Me | H | cyclohexyl | |
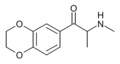 |
3,4-EDMC | 3,4-ethylenedioxy | Me | H | Me | 30253-44-2 |
 |
βk-IMP | 3,4-trimethylene | Me | H | Me | 100608-69-3 |
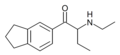 |
βk-IBP | 3,4-trimethylene | Et | H | Et | |
 |
βk-IVP | 3,4-trimethylene | nPr | H | Et | |
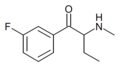 |
3-Fluorobuphedrone | 3-F | Et | H | Me | |
 |
4-Fluorobuphedrone | 4-F | Et | H | Me | 1368599-12-5 |
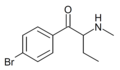 |
4-Bromobuphedrone | 4-Br | Et | H | Me | |
 |
3-Methylbuphedrone | 3-Me | Et | H | Me | 1797911-07-9 |
 |
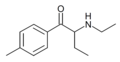
4-Me-MABP | 4-Me | Et | H | Me | 1336911-98-8 |
 |
4-Me-NEB | 4-Me | Et | H | Et | 18268-19-4 |
 |
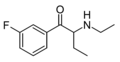
2-F-NEB | 2-F | Et | H | Et | |
 |
3F-NEB | 3-F | Et | H | Et | |
 |
4-F-NEB | 4-F | Et | H | Et | |
 |
4-Me-DMB | 4-Me | Et | Me | Me | |
 |
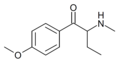
3,4-DMEB | 3,4-dimethyl | Et | H | Et | |
 |
4-Methoxybuphedrone | 4-MeO | Et | H | Me | |
 |
Butylone | 3,4-methylenedioxy | Et | H | Me | 802575-11-7 |
 |
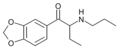
Eutylone | 3,4-methylenedioxy | Et | H | Et | 802855-66-9 |
 |
βk-PBDB | 3,4-methylenedioxy | Et | H | nPr | |
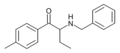 |
Bn-4-MeMABP | 4-Me | Et | H | Bn | 1445751-39-2 |
 |
BMDB | 3,4-methylenedioxy | Et | H | Bn | 1445751-47-2 |
 |
N-Cyclohexylbutylone | 3,4-methylenedioxy | Et | H | cyclohexyl | |
 |
βk-DMBDB | 3,4-methylenedioxy | Et | Me | Me | 802286-83-5 |
 |
βk-MMDMA | 3,4-methylenedioxy-5-MeO | Me | H | Me | 2230716-98-8 |
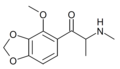 |
βk-MMDMA-2 | 2-MeO-3,4-methylenedioxy | Me | H | Me | |
 |
βk-DMMDA | 2,5-diMeO-3,4-methylenedioxy | Me | H | H | |
 |
5-Methylmethylone | 3,4-methylenedioxy-5-Me | Me | H | Me | 1364933-83-4 |
 |
5-Methylethylone | 3,4-methylenedioxy-5-Me | Me | H | Et | 1364933-82-3 |
 |
2-Methylbutylone | 2-Me-3,4-methylenedioxy | Et | H | Me | 1364933-86-7 |
 |
5-Methylbutylone | 3,4-methylenedioxy-5-Me | Et | H | Me | 1354631-29-0 |
 |
Pentylone | 3,4-methylenedioxy | nPr | H | Me | 698963-77-8 |
 |
N-Ethylpentylone | 3,4-methylenedioxy | nPr | H | Et | 727641-67-0 |
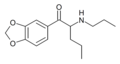 |
N-propylpentylone | 3,4-methylenedioxy | nPr | H | nPr | |
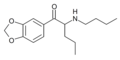 |
N-butylpentylone | 3,4-methylenedioxy | nPr | H | nBu | |
 |
2,3-Dipentylone | 2,3-methylenedioxy | nPr | Me | Me | |
 |
Dipentylone | 3,4-methylenedioxy | nPr | Me | Me | 17763-13-2 |
 |
N,N-Diethylnorpentylone | 3,4-methylenedioxy | nPr | Et | Et | |
 |
Hexylone | 3,4-methylenedioxy | nBu | H | Me | |
 |
Isohexylone | 3,4-methylenedioxy | iBu | H | Me | 1157947-89-1 |
 |
Isoheptylone | 3,4-methylenedioxy | iPe | H | Me | |
 |
N-Ethylhexylone | 3,4-methylenedioxy | nBu | H | Et | 27912-41-0 |
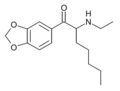 |
N-Ethylheptylone | 3,4-methylenedioxy | nPe | H | Et | |
 |
4-MEAP | 4-Me | nPr | H | Et | 746540-82-9 |
 |
3,4-DMEP | 3,4-dimethyl | nPr | H | Et | |
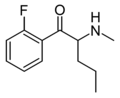 |
2-F-Pentedrone | 2-F | nPr | H | Me | |
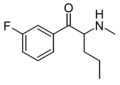 |
3-F-Pentedrone | 3-F | nPr | H | Me | |
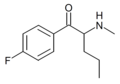 |
4-F-Pentedrone | 4-F | nPr | H | Me | |
 |
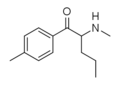
4-Cl-Pentedrone | 4-Cl | nPr | H | Me | 2167949-43-9 |
 |
4-Methylpentedrone | 4-Me | nPr | H | Me | 1373918-61-6 |
 |
DL-4662 | 3,4-dimethoxy | nPr | H | Et | 1674389-55-9 |
 |
4-F-iPr-norpentedrone | 4-F | nPr | H | iPr | |
 |
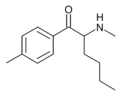
3-CBV | 3-Cl | nPr | H | tBu | |
 |
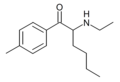
4-methylhexedrone | 4-Me | nBu | H | Me | |
 |
MEH | 4-Me | nBu | H | Et | |
 |
3F-NEH | 3-F | nBu | H | Et | |
 |
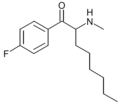
4-F-hexedrone | 4-F | nBu | H | Me | |
 |
4-F-octedrone | 4-F | hexyl | H | Me | |
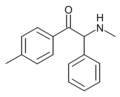 |
α-phenylmephedrone | 4-Me | phenyl | H | Me | |
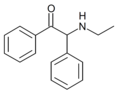 |
βk-Ephenidine | H | phenyl | H | Et | 22312-16-9 |
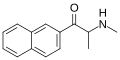 |
BMAPN | β-naphthyl instead of phenyl | Me | H | Me | |
 |
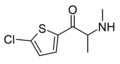
βk-Methiopropamine | thiophen-2-yl instead of phenyl | Me | H | Me | 24065-17-6 |
 |
5-Cl-bk-MPA | 5-chlorothiophen-2-yl instead of phenyl | Me | H | Me | |
 |
βk-5-MAPB | benzofuran-5-yl instead of phenyl | Me | H | Me | |
 |
βk-6-MAPB | benzofuran-6-yl instead of phenyl | Me | H | Me | |
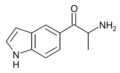 |
βk-5-IT | indol-5-yl instead of phenyl | Me | H | H | 1369231-36-6 |
 |
βk-5F-NM-AMT[39] | 5-fluoroindol-3-yl instead of phenyl | Me | H | Me | |
 |
α-Phthalimidopropiophenone | H | Me | phthalimido | 19437-20-8 | |
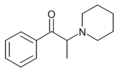 |
PPPO | H | Me | piperidinyl | ||
 |
PPBO | H | Et | piperidinyl | 92728-82-0 | |
 |
FPPVO | 4-F | nPr | piperidinyl | ||
 |
3,4-Pr-PipVP | 3,4-trimethylene | nPr | piperidinyl | ||
 |
MDPV-azepane | 3,4-methylenedioxy | nPr | azepane | ||
 |
Caccure 907 | 4-SCH3 | α,α-di-Me | morpholinyl | ||
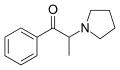 |
α-PPP | H | Me | pyrrolidinyl | 19134-50-0 | |
 |
α-PBP | H | Et | pyrrolidinyl | 13415-54-8 | |
 |
α-PVP (O-2387) | H | nPr | pyrrolidinyl | 14530-33-7 | |
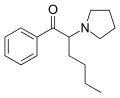 |
α-PHP | H | nBu | pyrrolidinyl | 13415-86-6 | |
 |
α-PHiP | H | iBu | pyrrolidinyl | ||
 |
α-PEP (α-PHPP) | H | nPe | pyrrolidinyl | 13415-83-3 | |
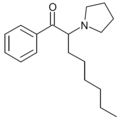 |
α-POP | H | hexyl | pyrrolidinyl | ||
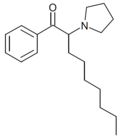 |
α-PNP | H | heptyl | pyrrolidinyl | ||
 |
DPPE (Alpha-D2PV) | H | phenyl | pyrrolidinyl | 27590-61-0 | |
 |
α-PcPeP | H | cyclopentyl | pyrrolidinyl | ||
 |
α-PCYP | H | cyclohexyl | pyrrolidinyl | 1803168-11-7 | |
 |
2-MePPP | 2-Me | Me | pyrrolidinyl | 2092429-83-7 | |
 |
3-MePPP | 3-Me | Me | pyrrolidinyl | 1214940-01-8 | |
 |
4-MePPP | 4-Me | Me | pyrrolidinyl | 1313393-58-6 | |
 |
3-MeO-PPP | 3-MeO | Me | pyrrolidinyl | ||
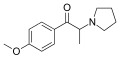 |
MOPPP | 4-MeO | Me | pyrrolidinyl | 478243-09-3 | |
 |
3-F-PPP | 3-F | Me | pyrrolidinyl | 1214939-99-7 | |
 |
FPPP | 4-F | Me | pyrrolidinyl | 28117-76-2 | |
 |
Cl-PPP | 4-Cl | Me | pyrrolidinyl | 93307-24-5 | |
 |
3-Br-PPP | 3-Br | Me | pyrrolidinyl | ||
 |
Br-PPP | 4-Br | Me | pyrrolidinyl | ||
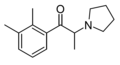 |
2,3-DMPPP | 2,3-dimethyl | Me | pyrrolidinyl | ||
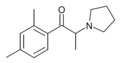 |
2,4-DMPPP | 2,4-dimethyl | Me | pyrrolidinyl | ||
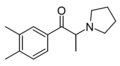 |
3,4-DMPPP | 3,4-dimethyl | Me | pyrrolidinyl | ||
 |
3-MPBP | 3-Me | Et | pyrrolidinyl | 1373918-60-5 | |
 |
3-F-PBP | 3-F | Et | pyrrolidinyl | 1373918-59-2 | |
 |
MPBP | 4-Me | Et | pyrrolidinyl | 732180-91-5 | |
 |
FPBP | 4-F | Et | pyrrolidinyl | 1373918-67-2 | |
 |
EPBP | 4-Et | Et | pyrrolidinyl | ||
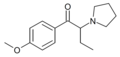 |
MOPBP | 4-MeO | Et | pyrrolidinyl | ||
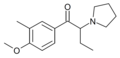 |
MMOPBP | 3-Me-4-MeO | Et | pyrrolidinyl | ||
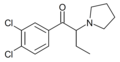 |
O-2384 | 3,4-dichloro | Et | pyrrolidinyl | 850352-65-7 | |
 |
2-Me-PVP | 2-Me | nPr | pyrrolidinyl | 850352-54-4 | |
 |
3-Me-PVP | 3-Me | nPr | pyrrolidinyl | 13415-85-5 | |
 |
Pyrovalerone (O-2371) | 4-Me | nPr | pyrrolidinyl | 3563-49-3 | |
 |
4-Et-PVP | 4-Et | nPr | pyrrolidinyl | ||
 |
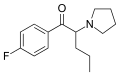
3F-PVP | 3-F | nPr | pyrrolidinyl | 2725852-55-9 | |
 |
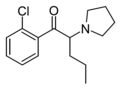
FPVP | 4-F | nPr | pyrrolidinyl | 850352-31-7 | |
 |
2-Cl-PVP | 2-Cl | nPr | pyrrolidinyl | ||
 |
3-Cl-PVP | 3-Cl | nPr | pyrrolidinyl | ||
 |
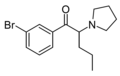
4-Cl-PVP | 4-Cl | nPr | pyrrolidinyl | 5537-17-7 | |
 |
3-Br-PVP | 3-Br | nPr | pyrrolidinyl | ||
 |
4-Br-PVP | 4-Br | nPr | pyrrolidinyl | ||
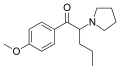 |
MOPVP | 4-MeO | nPr | pyrrolidinyl | 5537-19-9 | |
 |
DMOPVP | 3,4-dimethoxy | nPr | pyrrolidinyl | 850442-84-1 | |
 |
DMPVP | 3,4-dimethyl | nPr | pyrrolidinyl | ||
 |
O-2390 | 3,4-dichloro | nPr | pyrrolidinyl | 850352-61-3 | |
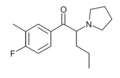 |
MFPVP | 3-methyl-4-fluoro | nPr | pyrrolidinyl | ||
 |
MPHP | 4-Me | nBu | pyrrolidinyl | 34138-58-4 | |
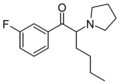 |
3F-PHP | 3-F | nBu | pyrrolidinyl | ||
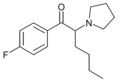 |
4F-PHP | 4-F | nBu | pyrrolidinyl | 2230706-09-7 | |
 |
4-Cl-PHP | 4-Cl | nBu | pyrrolidinyl | 2748592-29-0 | |
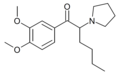 |
DMOPHP | 3,4-dimethoxy | nBu | pyrrolidinyl | ||
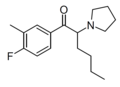 |
MFPHP | 3-Me-4-F | nBu | pyrrolidinyl | ||
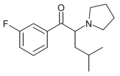 |
3F-PiHP | 3-F | iBu | pyrrolidinyl | ||
 |
4F-PiHP | 4-F | iBu | pyrrolidinyl | ||
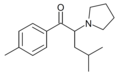 |
O-2394 | 4-Me | iBu | pyrrolidinyl | 850352-51-1 | |
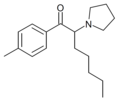 |
MPEP | 4-Me | pentyl | pyrrolidinyl | ||
 |
4F-PV8 | 4-F | pentyl | pyrrolidinyl | ||
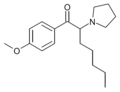 |
4-MeO-PV8 | 4-MeO | pentyl | pyrrolidinyl | ||
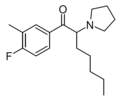 |
MFPEP | 3-Me-4-F | pentyl | pyrrolidinyl | ||
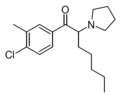 |
MCPEP | 3-Me-4-Cl | pentyl | pyrrolidinyl | ||
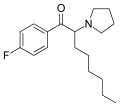 |
4F-PV9 | 4-F | hexyl | pyrrolidinyl | ||
 |
4-MeO-PV9 | 4-MeO | hexyl | pyrrolidinyl | ||
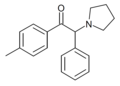 |
α-Phenylpyrovalerone | 4-Me | phenyl | pyrrolidinyl | ||
 |
MDPPP | 3,4-methylenedioxy | Me | pyrrolidinyl | 783241-66-7 | |
 |
MDMPP | 3,4-methylenedioxy | α,α-di-Me | pyrrolidinyl | ||
 |
MDPBP | 3,4-methylenedioxy | Et | pyrrolidinyl | 784985-33-7 | |
 |
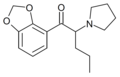
MDPV | 3,4-methylenedioxy | nPr | pyrrolidinyl | 687603-66-3 | |
 |
2,3-MDPV | 2,3-methylenedioxy | nPr | pyrrolidinyl | ||
 |
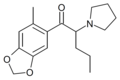
5-Me-MDPV | 3,4-methylenedioxy-5-Me | nPr | pyrrolidinyl | ||
 |
6-Me-MDPV | 2-Me-4,5-methylenedioxy | nPr | pyrrolidinyl | ||
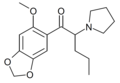 |
6-MeO-MDPV | 2-MeO-4,5-methylenedioxy | nPr | pyrrolidinyl | ||
 |
Br-MeO-MDPV | 2,3-methylenedioxy-4-MeO-5-Br | nPr | pyrrolidinyl | ||
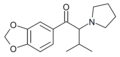 |
MDPiVP | 3,4-methylenedioxy | iPr | pyrrolidinyl | ||
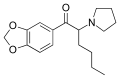 |
MDPHP | 3,4-methylenedioxy | nBu | pyrrolidinyl | 776994-64-0 | |
 |
MDPHiP | 3,4-methylenedioxy | iBu | pyrrolidinyl | ||
 |
MDPEP (MD-PV8) | 3,4-methylenedioxy | pentyl | pyrrolidinyl | 24646-39-7 | |
 |
MDPOP (MD-PV9) | 3,4-methylenedioxy | hexyl | pyrrolidinyl | 24646-40-0 | |
 |
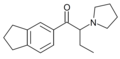
3,4-EtPV | 3,4-dimethylene | nPr | pyrrolidinyl | ||
 |
5-PPDi | 3,4-trimethylene | Et | pyrrolidinyl | ||
 |
Indanyl-α-PVP | 3,4-trimethylene | nPr | pyrrolidinyl | 2748590-83-0 | |
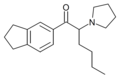 |
5-BPDi | 3,4-trimethylene | nBu | pyrrolidinyl | ||
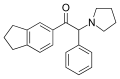 |
IPPV | 3,4-trimethylene | phenyl | pyrrolidinyl | ||
 |
TH-PBP | 3,4-tetramethylene | Et | pyrrolidinyl | ||
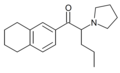 |
TH-PVP | 3,4-tetramethylene | nPr | pyrrolidinyl | 2304915-07-7 | |
 |
TH-PHP | 3,4-tetramethylene | nBu | pyrrolidinyl | ||
 |
5-DBFPV | 2,3-dihydrobenzofuran-5-yl instead of Ph | nPr | pyrrolidinyl | 1620807-94-4 | |
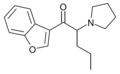 |
3-BF-PVP | benzofuran-3-yl instead of Ph | nPr | pyrrolidinyl | ||
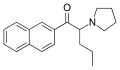 |
Naphyrone (O-2482) | β-naphthyl instead of phenyl | nPr | pyrrolidinyl | 850352-53-3 | |
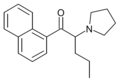 |
α-Naphyrone | α-naphthyl instead of phenyl | nPr | pyrrolidinyl | ||
 |
α-PPT | thiophen-2-yl instead of phenyl | Me | pyrrolidinyl | ||
 |
α-PBT | thiophen-2-yl instead of phenyl | Et | pyrrolidinyl | ||
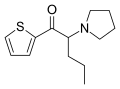 |
α-PVT | thiophen-2-yl instead of phenyl | nPr | pyrrolidinyl | 1400742-66-6 | |
Legality
[edit]On 2 April 2010, the Advisory Council on the Misuse of Drugs in the UK announced that a broad structure-based ban of this entire class of compounds would be instituted, following extensive publicity around grey-market sales and recreational use of mephedrone, a common member of the family. This ban covers compounds with the aforementioned general structure, with 28 compounds specifically named.[40]
"Any compound (not being bupropion or a substance for the time being specified in paragraph 2.2) structurally derived from 2-amino-1-phenyl-1-propanone by modification in any of the following ways, that is to say,
(i) by substitution in the phenyl ring to any extent with alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents, whether or not further substituted in the phenyl ring by one or more other univalent substituents;
(ii) by substitution at the 3-position with an alkyl substituent;
(iii) by substitution at the nitrogen atom with alkyl or dialkyl groups, or by inclusion of the nitrogen atom in a cyclic structure."
— ACMD, 2 April 2010
This text was added as an amendment to the Misuse of Drugs Act 1971, to come into force on 16 April 2010.[41] Note that four of the above compounds (cathinone, methcathinone, diethylpropion and pyrovalerone) were already illegal in the UK at the time the ACMD report was issued. Two compounds were specifically excluded from the ban, these being bupropion because of its common use in medicine and relative lack of abuse potential, and naphyrone because its structure falls outside the generic definition and not enough evidence was yet available to justify a ban.
Naphyrone analogues were subsequently banned in July 2010 following a further review by the ACMD,[42][43] along with a further broad based structure ban even more expansive than the last.[44][45]
"Any compound structurally derived from 2–aminopropan–1–one by substitution at the 1-position with any monocyclic, or fused-polycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), whether or not the compound is
further modified in any of the following ways, that is to say—
(i) by substitution in the ring system to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents;
(ii) by substitution at the 3–position with an alkyl substituent;
(iii) by substitution at the 2-amino nitrogen atom with alkyl or dialkyl groups, or
by inclusion of the 2-amino nitrogen atom in a cyclic structure".
— Home Office, 13 July 2010.

The substitutions in the general structure for naphyrone analogues subject to the ban may be described as follows:
- Cyc = any monocyclic, or fused-polycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), including analogues where the ring system is substituted to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents
- R1 = hydrogen or any alkyl group
- R2 = hydrogen, any alkyl group, or incorporation in a cyclic structure
- R3 = hydrogen, any alkyl group, or incorporation in a cyclic structure
More new derivatives have however continued to appear, with the UK reporting more novel cathinone derivatives detected in 2010 than any other country in Europe, with most of them first identified after the generic ban had gone into effect and thus already being illegal despite never having been previously reported.[46]
In the United States, substituted cathinones are the psychoactive ingredients in "bath salts" which as of July 2011 were banned by at least 28 states, but not by the federal government.[47]
See also
[edit]- Markush structure
- Substituted amphetamines
- Substituted β-hydroxyamphetamines
- Substituted methylenedioxyphenethylamines
- Substituted phenethylamines
- Substituted phenylmorpholines
- Structural scheduling of synthetic cannabinoids
- Arylcyclohexylamines
- List of aminorex analogues
- List of fentanyl analogues
- List of methylphenidate analogues
References
[edit]- ^ Meltzer PC, Butler D, Deschamps JR, Madras BK (February 2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors". Journal of Medicinal Chemistry. 49 (4): 1420–32. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278.
- ^ Paillet-Loilier M, Cesbron A, Le Boisselier R, Bourgine J, Debruyne D (2014). "Emerging drugs of abuse: current perspectives on substituted cathinones". Substance Abuse and Rehabilitation. 5: 37–52. doi:10.2147/SAR.S37257. PMC 4043811. PMID 24966713.
- ^ Simmons SJ, Leyrer-Jackson JM, Oliver CF, Hicks C, Muschamp JW, Rawls SM, Olive MF (October 2018). "DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants". ACS Chemical Neuroscience. 9 (10): 2379–2394. doi:10.1021/acschemneuro.8b00147. PMC 6197900. PMID 29714473.
- ^ Beck O, Bäckberg M, Signell P, Helander A (April 2018). "Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats". Clinical Toxicology. 56 (4): 256–263. doi:10.1080/15563650.2017.1370097. PMID 28895757. S2CID 3401681.
- ^ Majchrzak M, Celiński R, Kuś P, Kowalska T, Sajewicz M (2018). "The newest cathinone derivatives as designer drugs: an analytical and toxicological review". Forensic Toxicology. 36 (1): 33–50. doi:10.1007/s11419-017-0385-6. PMC 5754390. PMID 29367861.
- ^ Colzato LS, Ruiz MJ, van den Wildenberg WP, Hommel B (2011). "Khat use is associated with impaired working memory and cognitive flexibility". PLOS ONE. 6 (6): e20602. Bibcode:2011PLoSO...620602C. doi:10.1371/journal.pone.0020602. PMC 3115937. PMID 21698275.
- ^ Rothman RB, Baumann MH (2003). "Monoamine transporters and psychostimulant drugs". Eur. J. Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, Partilla JS, Rothman RB, Katz JL (February 2015). "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug and Alcohol Dependence. 147: 1–19. doi:10.1016/j.drugalcdep.2014.12.005. PMC 4297708. PMID 25548026.
- ^ Rothman RB, Baumann MH (December 2005). "Targeted screening for biogenic amine transporters: potential applications for natural products". Life Sciences. 78 (5): 512–518. doi:10.1016/j.lfs.2005.09.001. PMID 16202429.
- ^ Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (April 2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ a b Kuropka P, Zawadzki M, Szpot P (May 2023). "A narrative review of the neuropharmacology of synthetic cathinones-Popular alternatives to classical drugs of abuse". Hum Psychopharmacol. 38 (3): e2866. doi:10.1002/hup.2866. PMID 36866677.
Another feature that distinguishes [substituted cathinones (SCs)] from amphetamines is their negligible interaction with the trace amine associated receptor 1 (TAAR1). Activation of this receptor reduces the activity of dopaminergic neurones, thereby reducing psychostimulatory effects and addictive potential (Miller, 2011; Simmler et al., 2016). Amphetamines are potent agonists of this receptor, making them likely to self‐inhibit their stimulating effects. In contrast, SCs show negligible activity towards TAAR1 (Kolaczynska et al., 2021; Rickli et al., 2015; Simmler et al., 2014, 2016). [...] The lack of self‐regulation by TAAR1 may partly explain the higher addictive potential of SCs compared to amphetamines (Miller, 2011; Simmler et al., 2013).
- ^ a b Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME (April 2016). "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1". J Pharmacol Exp Ther. 357 (1): 134–144. doi:10.1124/jpet.115.229765. PMID 26791601.
- ^ Simmler LD, Rickli A, Hoener MC, Liechti ME (April 2014). "Monoamine transporter and receptor interaction profiles of a new series of designer cathinones". Neuropharmacology. 79: 152–160. doi:10.1016/j.neuropharm.2013.11.008. PMID 24275046.
- ^ Pottie E, Cannaert A, Stove CP (October 2020). "In vitro structure-activity relationship determination of 30 psychedelic new psychoactive substances by means of β-arrestin 2 recruitment to the serotonin 2A receptor". Arch Toxicol. 94 (10): 3449–3460. doi:10.1007/s00204-020-02836-w. hdl:1854/LU-8687071. PMID 32627074.
- ^ Europol 2008 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2011 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2013 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2017 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ European Monitoring Center for Drugs and Drug Addiction (December 2020). New psychoactive substances: global markets, glocal threats and the COVID-19 pandemic. An update from the EU Early Warning System (PDF). Luxembourg: Publications Office of the European Union. doi:10.2810/921262. ISBN 9789294975584.
- ^ Maurer HH, Kraemer T, Springer D, Staack RF (April 2004). "Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis". Therapeutic Drug Monitoring. 26 (2): 127–31. doi:10.1097/00007691-200404000-00007. PMID 15228152. S2CID 9255084.
- ^ Davis S, Rands-Trevor K, Boyd S, Edirisinghe M (April 2012). "The characterisation of two halogenated cathinone analogues: 3,5-difluoromethcathinone and 3,5-dichloromethcathinone". Forensic Science International. 217 (1–3): 139–45. doi:10.1016/j.forsciint.2011.10.042. PMID 22088945.
- ^ Liu C, Jia W, Li T, Hua Z, Qian Z (August 2017). "Identification and analytical characterization of nine synthetic cathinone derivatives N-ethylhexedrone, 4-Cl-pentedrone, 4-Cl-α-EAPP, propylone, N-ethylnorpentylone, 6-MeO-bk-MDMA, α-PiHP, 4-Cl-α-PHP, and 4-F-α-PHP". Drug Testing and Analysis. 9 (8): 1162–1171. doi:10.1002/dta.2136. PMID 27863142.
- ^ Błażewicz A, Bednarek E, Popławska M, Olech N, Sitkowski J, Kozerski L (2019). "Identification and structural characterization of synthetic cathinones: N-propylcathinone, 2,4-dimethylmethcathinone, 2,4-dimethylethcathinone, 2,4-dimethyl-α-pyrrolidinopropiophenone, 4-bromo-α-pyrrolidinopropiophenone, 1-(2,3-dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)hexan-1-one and 2,4-dimethylisocathinone". Forensic Toxicol. 37 (2): 288–307. doi:10.1007/s11419-018-00463-w. S2CID 59618061.
- ^ Westphal F, Girreser U, Angerer V, Auwärter V (January 2016). "Analytische Daten neuer 2-aminosubstituierter Methylendioxyvalerophenonderivate". Toxichem Krimtech. 83 (1): 3–29.
- ^ Majchrzak M, Celiński R, Kuś P, Kowalska T, Sajewicz M (2018). "The newest cathinone derivatives as designer drugs: an analytical and toxicological review". Forensic Toxicology. 36 (1): 33–50. doi:10.1007/s11419-017-0385-6. PMC 5754390. PMID 29367861.
- ^ Carlsson A, Sandgren V, Svensson S, Konradsson P, Dunne S, Josefsson M, Dahlén J (February 2018). "Prediction of designer drugs: Synthesis and spectroscopic analysis of synthetic cathinone analogs that may appear on the Swedish drug market". Drug Testing and Analysis. 10 (7): 1076–1098. doi:10.1002/dta.2366. PMID 29426062.
- ^ Cheng WC, Wong WC (May 2019). "Forensic drug analysis of chloro-N,N-dimethylcathinone (CDC) and chloroethcathinone (CEC): Identification of 4-CDC and 4-CEC in drug seizures and differentiation from their ring-substituted positional isomers". Forensic Science International. 298: 268–277. doi:10.1016/j.forsciint.2019.03.002. PMID 30925345. S2CID 87589412.
- ^ Lajtai A, Mayer M, Lakatos Á, Kuzma M, Miseta A (November 2020). "New psychoactive versus conventional stimulants - a ten-year review of casework in Hungary". Legal Medicine. 47: 101780. doi:10.1016/j.legalmed.2020.101780. PMID 32882537. S2CID 221496728.
- ^ Jones NS, Comparin JH (2020). "Interpol review of controlled substances 2016-2019". Forensic Science International. Synergy. 2: 608–669. doi:10.1016/j.fsisyn.2020.01.019. PMC 7770462. PMID 33385148.
- ^ "Valtioneuvoston asetus kuluttajamarkkinoilta kielletyistä psykoaktiivisista aineista" [Government Decree on Psychoactive Substances Banned from the Consumer Market]. Finlex Data Bank (in Finnish).
- ^ Baggott M. Advantageous Tryptamine Compositions For Mental Disorders or Enhancement. Patent WO 2022/061242
- ^ Advisory Council on the Misuse of Drugs (UK). Consideration of the cathinones. 31 March 2010. Archived 22 September 2011 at the Wayback Machine Retrieved 2011-07-17.
- ^ "The Misuse of Drugs (Amendment) (England, Wales and Scotland) Regulations 2010 No. 1144". Opsi.gov.uk. Retrieved 8 April 2010.
- ^ "NRG-1 'legal high' drug is banned". BBC News. 12 July 2010. Retrieved 17 July 2010.
- ^ "Advisory Council on the Misuse of Drugs Naphyrone Report (2010)". Home Office. 7 July 2010. Archived from the original on 17 July 2010. Retrieved 17 July 2010.
- ^ "Explanatory Memorandum To The Misuse of Drugs (Amendment No. 2) (England, Wales and Scotland) Regulations 2010 No. 1799" (PDF). Opsi.gov.uk. Retrieved 18 July 2010.
- ^ "The Misuse of Drugs (Amendment No. 2) (England, Wales and Scotland) Regulations 2010 No. 1799" (PDF). Opsi.gov.uk. Retrieved 18 July 2010.
- ^ European Monitoring Centre on Drugs and Drug Addiction. EMCDDA–Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA. Archived 14 March 2012 at the Wayback Machine Retrieved 2011-07-17.
- ^ Goodnough A, Zezima K (16 July 2011). "An Alarming New Stimulant, Legal in Many States". The New York Times. Retrieved 17 July 2011.
