25CN-NBOH
 | |
| Clinical data | |
|---|---|
| Other names | NBOH-2C-CN |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
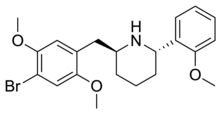
| Formula | C18H20N2O3 |
| Molar mass | 312.369 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
25CN-NBOH (sometimes also referred to as NBOH-2C-CN)[1] is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen.[2] It is a member of the NBOMe family of psychedelics.[3]
This compound is notable as one of the most selective agonists of the serotonin 5-HT2A receptor yet discovered, with an affinity (pKi) of 8.88 at the human serotonin 5-HT2A receptor, 100-fold selectivity for the serotonin 5-HT2A receptor over the serotonin 5-HT2C, and 46-fold selectivity for the serotonin 5-HT2A receptor over the serotonin 5-HT2B.[4][5][6][7] However, another study found that 25CN-NBOH only had around 25-fold selectivity for the serotonin 5-HT2A receptor over the serotonin 5-HT2B and 5-HT2C receptors.[3][7] In any case, in 2020, 25CN-NBOH and the related drug (S,S)-DMBMPP were described as the most selective serotonin 5-HT2A receptor agonists discovered to date.[3]
A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to serotonin 5-HT2 receptors and autoradiography.[8]
Structure
[edit]The structure of 25CN-NBOH in complex with an engineered Gαq heterotrimer of the 5-HT2AR has been determined by cryoelectron microscopy (cryo-EM), showing a distinct binding mode when compared to LSD.[9]
Synthesis
[edit]25CN-NBOH is readily available from 2C-H in 57% over 4 steps.[10]
Animal studies
[edit]25CN-NBOH was found to partially substitute for DOI but was considerably weaker at inducing a head-twitch response (HTR) in mice.[11][12] Another in vivo evaluation of 25CN-NBOH concluded that "Given its distinct in vitro selectivity for 5-HT2A over non 5-HT2 receptors and its behavioral dynamics, 25CN-NBOH appears to be a powerful tool for dissection of receptor-specific cortical circuit dynamics, including 5-HT2A related psychoactivity."[13]
25CN-NBOH induces the HTRs also refererred to as "wet dog shakes" in rodents and the cortical fingerprint of serotonin-2A-receptor-mediated shaking behavior has been investigated in detail.[14]
Additional in vivo investigations with this ligand has emerged.[15][16][17][18][19][20][21][22] Chronic administration in mice lead to desensitization of the 5-HT2AR (measured via HTR) and increased startle amplitude[23] whereas it does not effect reversal learning in mice.[24] 25CN-NBOH was shown to increase the production of CTGF in chondrocytes.[25] In rats, 25CN-NBOH induce a reduction in conditioned fear that was countered by pretreatment with 5-HT2AR inverse agonist MDL100907.[26]
A bioanalytical method for the detection of 25CN-NBOH has been developed.[27]
Literature
[edit]A review covering the literature up to 2020 was published in 2021.[28]
Related compounds
[edit]The tendency of the 4-cyano substitution to confer high 5-HT2A selectivity had previously been observed with DOCN,[29] but this was not sufficiently potent to be widely adopted as a research ligand. 25CN-NBOH is still slightly less selective for 5-HT2A than the more complex cyclised derivative 2S,6S-DMBMPP ((2S,6S)-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine),[30] in binding assays, however it is also less complex to synthesise and has higher efficacy and selectivity in functional assays as a partial agonist of the 5-HT2A receptor.
Legality
[edit]Hungary
[edit]25CN-NBOH is illegal in Hungary.[31]
United Kingdom
[edit]This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[32]
See also
[edit]References
[edit]- ^ "25CN-NBOH". Chemical Probes.
- ^ "CNS Medicinal Chemistry in the Kristensen Group". Department of Drug Design and Pharmacology. University of Copenhagen. 25 March 2019.
- ^ a b c Poulie CB, Jensen AA, Halberstadt AL, Kristensen JL (December 2020). "DARK Classics in Chemical Neuroscience: NBOMes". ACS Chem Neurosci. 11 (23): 3860–3869. doi:10.1021/acschemneuro.9b00528. PMC 9191638. PMID 31657895.
- ^ Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–249. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- ^ Jensen AA, McCorvy JD, Leth-Petersen S, Bundgaard C, Liebscher G, Kenakin TP, et al. (June 2017). "Detailed Characterization of the In Vitro Pharmacological and Pharmacokinetic Properties of N-(2-Hydroxybenzyl)-2,5-Dimethoxy-4-Cyanophenylethylamine (25CN-NBOH), a Highly Selective and Brain-Penetrant 5-HT2A Receptor Agonist". The Journal of Pharmacology and Experimental Therapeutics. 361 (3): 441–453. doi:10.1124/jpet.117.239905. PMID 28360333.
- ^ Hansen M (2011). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen.
- ^ a b Halberstadt AL, Sindhunata IS, Scheffers K, Flynn AD, Sharp RF, Geyer MA, Young JW (August 2016). "Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice". Neuropharmacology. 107: 364–375. doi:10.1016/j.neuropharm.2016.03.038. PMC 5403251. PMID 27020041.
- ^ Jensen AA, Halberstadt AL, Märcher-Rørsted E, Odland AU, Chatha M, Speth N, et al. (July 2020). "The selective 5-HT2A receptor agonist 25CN-NBOH: Structure-activity relationship, in vivo pharmacology, and in vitro and ex vivo binding characteristics of [3H]25CN-NBOH". Biochemical Pharmacology. 177: 113979. doi:10.1016/j.bcp.2020.113979. PMID 32298690. S2CID 215802376.
- ^ Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE, et al. (September 2020). "Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor". Cell. 182 (6): 1574–1588.e19. doi:10.1016/j.cell.2020.08.024. PMC 7593816. PMID 32946782.
- ^ Märcher-Rørsted E, Nykodemová J, Kristensen JL (2021-06-08). "An improved, scalable synthesis of the selective serotonin 2A receptor agonist 25CN-NBOH". SynOpen. 05 (2): a–1524–4439. doi:10.1055/a-1524-4439. ISSN 2509-9396.
- ^ Fantegrossi WE, Gray BW, Bailey JM, Smith DA, Hansen M, Kristensen JL (March 2015). "Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT₂A receptors, in mice". Psychopharmacology. 232 (6): 1039–1047. doi:10.1007/s00213-014-3739-3. PMC 4339409. PMID 25224567.
- ^ Halberstadt AL, van der Zee JV, Chatha M, Geyer MA, Powell SB (February 2019). "Chronic treatment with a metabotropic mGlu2/3 receptor agonist diminishes behavioral response to a phenethylamine hallucinogen". Psychopharmacology. 236 (2): 821–830. doi:10.1007/s00213-018-5118-y. PMC 6778591. PMID 30448990.
- ^ Buchborn T, Lyons T, Knöpfel T (2018). "Tolerance and Tachyphylaxis to Head Twitches Induced by the 5-HT2A Agonist 25CN-NBOH in Mice". Frontiers in Pharmacology. 9: 17. doi:10.3389/fphar.2018.00017. PMC 5808243. PMID 29467649.
- ^ Buchborn T, Lyons T, Song C, Feilding A, Knöpfel T (May 2023). "Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging". International Journal of Molecular Sciences. 24 (11): 9463. doi:10.3390/ijms24119463. ISSN 1422-0067. PMC 10253917. PMID 37298417.
- ^ Harmon JL, Wills LP, McOmish CE, Demireva EY, Gingrich JA, Beeson CC, Schnellmann RG (April 2016). "5-HT2 Receptor Regulation of Mitochondrial Genes: Unexpected Pharmacological Effects of Agonists and Antagonists". The Journal of Pharmacology and Experimental Therapeutics. 357 (1): 1–9. doi:10.1124/jpet.115.228395. PMC 4809314. PMID 26787771.
- ^ Odland AU, Jessen L, Kristensen JL, Fitzpatrick CM, Andreasen JT (February 2021). "The 5-hydroxytryptamine 2A receptor agonists DOI and 25CN-NBOH decrease marble burying and reverse 8-OH-DPAT-induced deficit in spontaneous alternation". Neuropharmacology. 183: 107838. doi:10.1016/j.neuropharm.2019.107838. PMID 31693871. S2CID 207831116.
- ^ Yang GE, Tae HJ, Lee TK, Park YE, Cho JH, Kim DW, et al. (September 2019). "Risperidone Treatment after Transient Ischemia Induces Hypothermia and Provides Neuroprotection in the Gerbil Hippocampus by Decreasing Oxidative Stress". International Journal of Molecular Sciences. 20 (18): 4621. doi:10.3390/ijms20184621. PMC 6770640. PMID 31540405.
- ^ Amodeo DA, Hassan O, Klein L, Halberstadt AL, Powell SB (October 2020). "Acute serotonin 2A receptor activation impairs behavioral flexibility in mice". Behavioural Brain Research. 395: 112861. doi:10.1016/j.bbr.2020.112861. PMID 32814148.
- ^ Xing L, Kalebic N, Namba T, Vaid S, Wimberger P, Huttner WB (December 2020). "Serotonin Receptor 2A Activation Promotes Evolutionarily Relevant Basal Progenitor Proliferation in the Developing Neocortex". Neuron. 108 (6): 1113–1129.e6. doi:10.1016/j.neuron.2020.09.034. PMID 33080227. S2CID 224775595.
- ^ Elsilä LV, Korhonen N, Hyytiä P, Korpi ER (2020). "Acute Lysergic Acid Diethylamide Does Not Influence Reward-Driven Decision Making of C57BL/6 Mice in the Iowa Gambling Task". Frontiers in Pharmacology. 11: 602770. doi:10.3389/fphar.2020.602770. PMC 7745734. PMID 33343373.
- ^ Liu X, Zhu H, Gao H, Tian X, Tan B, Su R (April 2022). "Gs signaling pathway distinguishes hallucinogenic and nonhallucinogenic 5-HT2AR agonists induced head twitch response in mice". Biochemical and Biophysical Research Communications. 598: 20–25. doi:10.1016/j.bbrc.2022.01.113. PMID 35149433. S2CID 246548173.
- ^ Ilchibaeva T, Tsybko A, Zeug A, Müller FE, Guseva D, Bischoff S, et al. (August 2022). "Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes". Cells. 11 (15): 2384. doi:10.3390/cells11152384. PMC 9368268. PMID 35954229.
- ^ Tsybko AS, Ilchibaeva TV, Filimonova EA, Eremin DV, Popova NK, Naumenko VS (December 2020). "The Chronic Treatment With 5-HT2A Receptor Agonists Affects the Behavior and the BDNF System in Mice". Neurochemical Research. 45 (12): 3059–3075. doi:10.1007/s11064-020-03153-5. PMID 33095437. S2CID 225052555.
- ^ Odland AU, Kristensen JL, Andreasen JT (August 2021). "The selective 5-HT2A receptor agonist 25CN-NBOH does not affect reversal learning in mice". Behavioural Pharmacology. 32 (5): 448–452. doi:10.1097/FBP.0000000000000626. PMID 33595957. S2CID 231953516.
- ^ Hori A, Nishida T, Takashiba S, Kubota S, Takigawa M (2017-11-16). "Regulatory mechanism of CCN2 production by serotonin (5-HT) via 5-HT2A and 5-HT2B receptors in chondrocytes". PLOS ONE. 12 (11): e0188014. Bibcode:2017PLoSO..1288014H. doi:10.1371/journal.pone.0188014. PMC 5690650. PMID 29145495.
- ^ Hagsäter SM, Pettersson R, Pettersson C, Atanasovski D, Näslund J, Eriksson E (September 2021). "A Complex Impact of Systemically Administered 5-HT2A Receptor Ligands on Conditioned Fear". The International Journal of Neuropsychopharmacology. 24 (9): 749–757. doi:10.1093/ijnp/pyab040. PMC 8453278. PMID 34228806.
- ^ Breusova K, Ernstsen KG, Palner M, Linnet K, Kristensen JL, Kretschmann AC (May 2021). "A quantitative method for the selective 5-HT2A agonist 25CN-NBOH in rat plasma and brain". Journal of Pharmaceutical and Biomedical Analysis. 199: 114016. doi:10.1016/j.jpba.2021.114016. PMID 33784574. S2CID 232431316.
- ^ Märcher Rørsted E, Jensen AA, Kristensen JL (November 2021). "25CN-NBOH: A Selective Agonist for in vitro and in vivo Investigations of the Serotonin 2A Receptor". ChemMedChem. 16 (21): 3263–3270. doi:10.1002/cmdc.202100395. PMID 34288515. S2CID 236157499.
- ^ Nelson DL, Lucaites VL, Wainscott DB, Glennon RA (January 1999). "Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 359 (1): 1–6. doi:10.1007/PL00005315. PMID 9933142. S2CID 20150858.
- ^ Juncosa JI, Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, et al. (January 2013). "Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands". ACS Chemical Neuroscience. 4 (1): 96–109. doi:10.1021/cn3000668. PMC 3547484. PMID 23336049.
- ^ "A Magyarországon megjelent, a Kábítószer és Kábítószer-függőség Európai Megfigyelő Központjának Korai Jelzőrendszerébe (EMCDDA EWS) 2005 óta bejelentett ellenőrzött anyagok büntetőjogi vonatkozású besorolása" [The criminal law-related classification of controlled substances announced in 2005 in the Early Warning System of the European Monitoring Center for Drugs and Drug Addiction (EMCDDA EWS) in Hungary] (PDF) (in Hungarian). September 2015.
- ^ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Statutory Instruments 2014 No. 1106. www.legislation.gov.uk.
