Tubulin
| Tubulin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 kif1a head-microtubule complex structure in atp-form | |||||||||
| Identifiers | |||||||||
| Symbol | Tubulin | ||||||||
| Pfam | PF00091 | ||||||||
| Pfam clan | CL0442 | ||||||||
| InterPro | IPR003008 | ||||||||
| PROSITE | PDOC00201 | ||||||||
| SCOP2 | 1tub / SCOPe / SUPFAM | ||||||||
| |||||||||
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton.[1] It was discovered and named by Hideo Mōri in 1968.[2] Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.
In eukaryotes, there are six members of the tubulin superfamily, although not all are present in all species.[3][4] Both α and β tubulins have a mass of around 50 kDa and are thus in a similar range compared to actin (with a mass of ~42 kDa). In contrast, tubulin polymers (microtubules) tend to be much bigger than actin filaments due to their cylindrical nature.
Tubulin was long thought to be specific to eukaryotes. More recently, however, several prokaryotic proteins have been shown to be related to tubulin.[5][6][7][8]
Characterization
[edit]Tubulin is characterized by the evolutionarily conserved Tubulin/FtsZ family, GTPase protein domain.
This GTPase protein domain is found in all eukaryotic tubulin chains,[9] as well as the bacterial protein TubZ,[8] the archaeal protein CetZ,[10] and the FtsZ protein family widespread in bacteria and archaea.[5][11]
Function
[edit]Microtubules
[edit]
α- and β-tubulin polymerize into dynamic microtubules. In eukaryotes, microtubules are one of the major components of the cytoskeleton, and function in many processes, including structural support, intracellular transport, and DNA segregation.
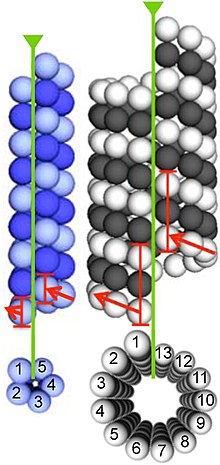
Microtubules are assembled from dimers of α- and β-tubulin. These subunits are slightly acidic, with an isoelectric point between 5.2 and 5.8.[14] Each has a molecular weight of approximately 50 kDa.[15]
To form microtubules, the dimers of α- and β-tubulin bind to GTP and assemble onto the (+) ends of microtubules while in the GTP-bound state.[16] The β-tubulin subunit is exposed on the plus end of the microtubule, while the α-tubulin subunit is exposed on the minus end. After the dimer is incorporated into the microtubule, the molecule of GTP bound to the β-tubulin subunit eventually hydrolyzes into GDP through inter-dimer contacts along the microtubule protofilament.[17] The GTP molecule bound to the α-tubulin subunit is not hydrolyzed during the whole process. Whether the β-tubulin member of the tubulin dimer is bound to GTP or GDP influences the stability of the dimer in the microtubule. Dimers bound to GTP tend to assemble into microtubules, while dimers bound to GDP tend to fall apart; thus, this GTP cycle is essential for the dynamic instability of the microtubule.
Bacterial microtubules
[edit]Homologs of α- and β-tubulin have been identified in the Prosthecobacter genus of bacteria.[6] They are designated BtubA and BtubB to identify them as bacterial tubulins. Both exhibit homology to both α- and β-tubulin.[18] While structurally highly similar to eukaryotic tubulins, they have several unique features, including chaperone-free folding and weak dimerization.[19] Cryogenic electron microscopy showed that BtubA/B forms microtubules in vivo, and suggested that these microtubules comprise only five protofilaments, in contrast to eukaryotic microtubules, which usually contain 13.[13] Subsequent in vitro studies have shown that BtubA/B forms four-stranded 'mini-microtubules'.[20]
DNA segregation
[edit]Cell division
[edit]Prokaryotic division
[edit]FtsZ is found in nearly all Bacteria and Archaea, where it functions in cell division, localizing to a ring in the middle of the dividing cell and recruiting other components of the divisome, the group of proteins that together constrict the cell envelope to pinch off the cell, yielding two daughter cells. FtsZ can polymerize into tubes, sheets, and rings in vitro, and forms dynamic filaments in vivo.
TubZ functions in segregating low copy-number plasmids during bacterial cell division. The protein forms a structure unusual for a tubulin homolog; two helical filaments wrap around one another.[21] This may reflect an optimal structure for this role since the unrelated plasmid-partitioning protein ParM exhibits a similar structure.[22]
Cell shape
[edit]CetZ functions in cell shape changes in pleomorphic Haloarchaea. In Haloferax volcanii, CetZ forms dynamic cytoskeletal structures required for differentiation from a plate-shaped cell form into a rod-shaped form that exhibits swimming motility.[10]
Types
[edit]Eukaryotic
[edit]The tubulin superfamily contains six families (alpha-(α), beta-(β), gamma-(γ), delta-(δ), epsilon-(ε), and zeta-(ζ) tubulins).[23]
α-Tubulin
[edit]Human α-tubulin subtypes include:[citation needed]
β-Tubulin
[edit]
All drugs that are known to bind to human tubulin bind to β-tubulin.[24] These include paclitaxel, colchicine, and the vinca alkaloids, each of which have a distinct binding site on β-tubulin.[24]
In addition, several anti-worm drugs preferentially target the colchicine site of β-Tubulin in worm rather than in higher eukaryotes. While mebendazole still retains some binding affinity to human and Drosophila β-tubulin,[25] albendazole almost exclusively binds to the β-tubulin of worms and other lower eukaryotes.[26][27]
Class III β-tubulin is a microtubule element expressed exclusively in neurons,[28] and is a popular identifier specific for neurons in nervous tissue. It binds colchicine much more slowly than other isotypes of β-tubulin.[29]
β1-tubulin, sometimes called class VI β-tubulin,[30] is the most divergent at the amino acid sequence level.[31] It is expressed exclusively in megakaryocytes and platelets in humans and appears to play an important role in the formation of platelets.[31] When class VI β-tubulin were expressed in mammalian cells, they cause disruption of microtubule network, microtubule fragment formation, and can ultimately cause marginal-band like structures present in megakaryocytes and platelets.[32]
Katanin is a protein complex that severs microtubules at β-tubulin subunits, and is necessary for rapid microtubule transport in neurons and in higher plants.[33]
Human β-tubulins subtypes include:[citation needed]
γ-Tubulin
[edit]
γ-Tubulin, another member of the tubulin family, is important in the nucleation and polar orientation of microtubules. It is found primarily in centrosomes and spindle pole bodies, since these are the areas of most abundant microtubule nucleation. In these organelles, several γ-tubulin and other protein molecules are found in complexes known as γ-tubulin ring complexes (γ-TuRCs), which chemically mimic the (+) end of a microtubule and thus allow microtubules to bind. γ-tubulin also has been isolated as a dimer and as a part of a γ-tubulin small complex (γTuSC), intermediate in size between the dimer and the γTuRC. γ-tubulin is the best understood mechanism of microtubule nucleation, but certain studies have indicated that certain cells may be able to adapt to its absence, as indicated by mutation and RNAi studies that have inhibited its correct expression. Besides forming a γ-TuRC to nucleate and organize microtubules, γ-tubulin can polymerize into filaments that assemble into bundles and meshworks.[34]
Human γ-tubulin subtypes include:
Members of the γ-tubulin ring complex:
δ and ε-Tubulin
[edit]Delta (δ) and epsilon (ε) tubulin have been found to localize at centrioles and may play a role in centriole structure and function, though neither is as well-studied as the α- and β- forms.
Human δ- and ε-tubulin genes include:[citation needed]
ζ-Tubulin
[edit]Zeta-tubulin (IPR004058) is present in many eukaryotes, but missing from others, including placental mammals. It has been shown to be associated with the basal foot structure of centrioles in multiciliated epithelial cells.[4]
Prokaryotic
[edit]BtubA/B
[edit]BtubA (Q8GCC5) and BtubB (Q8GCC1) are found in some bacterial species in the Verrucomicrobiota genus Prosthecobacter.[6] Their evolutionary relationship to eukaryotic tubulins is unclear, although they may have descended from a eukaryotic lineage by lateral gene transfer.[19][18] Compared to other bacterial homologs, they are much more similar to eukaryotic tubulins. In an assembled structure, BtubB acts like α-tubulin and BtubA acts like β-tubulin.[35]
FtsZ
[edit]Many bacterial and euryarchaeotal cells use FtsZ to divide via binary fission. All chloroplasts and some mitochondria, both organelles derived from endosymbiosis of bacteria, also use FtsZ.[36] It was the first prokaryotic cytoskeletal protein identified.
TubZ
[edit]TubZ (Q8KNP3; pBt156) was identified in Bacillus thuringiensis as essential for plasmid maintenance.[8] It binds to a DNA-binding protein called TubR (Q8KNP2; pBt157) to pull the plasmid around.[37]
CetZ
[edit]CetZ (D4GVD7) is found in the euryarchaeal clades of Methanomicrobia and Halobacteria, where it functions in cell shape differentiation.[10]
Phage tubulins
[edit]Phages of the genus Phikzlikevirus, as well as a Serratia phage PCH45, use a shell protein (Q8SDA8) to build a nucleus-like structure called the phage nucleus. This structure encloses DNA as well as replication and transcription machinery. It protects phage DNA from host defenses like restriction enzymes and type I CRISPR-Cas systems. A spindle-forming tubulin, variously named PhuZ (B3FK34) and gp187, centers the nucleus in the cell.[38][39]
Odinarchaeota tubulin
[edit]Asgard archaea tubulin from hydrothermal-living Odinarchaeota (OdinTubulin) was identified as a genuine tubulin. OdinTubulin forms protomers and protofilaments most similar to eukaryotic microtubules, yet assembles into ring systems more similar to FtsZ, indicating that OdinTubulin may represent an evolution intermediate between FtsZ and microtubule-forming tubulins.[40]
Pharmacology
[edit]Tubulins are targets for anticancer drugs[41][42][43] such as vinblastine and vincristine,[44][45] and paclitaxel.[46] The anti-worm drugs mebendazole and albendazole as well as the anti-gout agent colchicine bind to tubulin and inhibit microtubule formation. While the former ultimately lead to cell death in worms, the latter arrests neutrophil motility and decreases inflammation in humans. The anti-fungal drug griseofulvin targets microtubule formation and has applications in cancer treatment.
Post-translational modifications
[edit]When incorporated into microtubules, tubulin accumulates a number of post-translational modifications, many of which are unique to these proteins. These modifications include detyrosination, acetylation, polyglutamylation, polyglycylation, phosphorylation, ubiquitination, sumoylation, and palmitoylation. Tubulin is also prone to oxidative modification and aggregation during, for example, acute cellular injury.[47]
Nowadays there are many scientific investigations of the acetylation done in some microtubules, specially the one by α-tubulin N-acetyltransferase (ATAT1) which is being demonstrated to play an important role in many biological and molecular functions and, therefore, it is also associated with many human diseases, specially neurological diseases.
See also
[edit]References
[edit]- ^ Gunning PW, Ghoshdastider U, Whitaker S, Popp D, Robinson RC (June 2015). "The evolution of compositionally and functionally distinct actin filaments". Journal of Cell Science. 128 (11): 2009–19. doi:10.1242/jcs.165563. PMID 25788699.
- ^ Mohri, H. (1968-03-16). "Amino-acid composition of "Tubulin" constituting microtubules of sperm flagella". Nature. 217 (5133): 1053–1054. doi:10.1038/2171053a0. ISSN 0028-0836. PMID 4296139.
- ^ Findeisen P, Mühlhausen S, Dempewolf S, Hertzog J, Zietlow A, Carlomagno T, Kollmar M "Six subgroups and extensive recent duplications characterize the evolution of the eukaryotic tubulin protein family" Genome Biol Evol (2014) 6:2274-2288.
- ^ a b Turk E, Wills AA, Kwon T, Sedzinski J, Wallingford JB, Stearns T "Zeta-Tubulin Is a Member of a Conserved Tubulin Module and Is a Component of the Centriolar Basal Foot in Multiciliated Cells" Current Biology (2015) 25:2177-2183.
- ^ a b Nogales E, Downing KH, Amos LA, Löwe J (June 1998). "Tubulin and FtsZ form a distinct family of GTPases". Nature Structural Biology. 5 (6): 451–8. doi:10.1038/nsb0698-451. PMID 9628483. S2CID 5945125.
- ^ a b c Jenkins C, Samudrala R, Anderson I, Hedlund BP, Petroni G, Michailova N, et al. (December 2002). "Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter". Proceedings of the National Academy of Sciences of the United States of America. 99 (26): 17049–54. Bibcode:2002PNAS...9917049J. doi:10.1073/pnas.012516899. PMC 139267. PMID 12486237.
- ^ Yutin N, Koonin EV (March 2012). "Archaeal origin of tubulin". Biology Direct. 7: 10. doi:10.1186/1745-6150-7-10. PMC 3349469. PMID 22458654.
- ^ a b c Larsen RA, Cusumano C, Fujioka A, Lim-Fong G, Patterson P, Pogliano J (June 2007). "Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis". Genes & Development. 21 (11): 1340–52. doi:10.1101/gad.1546107. PMC 1877747. PMID 17510284.
- ^ Nogales E, Wolf SG, Downing KH (January 1998). "Structure of the alpha beta tubulin dimer by electron crystallography". Nature. 391 (6663): 199–203. Bibcode:1998Natur.391..199N. doi:10.1038/34465. PMID 9428769. S2CID 4412367.
- ^ a b c Duggin IG, Aylett CH, Walsh JC, Michie KA, Wang Q, Turnbull L, et al. (March 2015). "CetZ tubulin-like proteins control archaeal cell shape". Nature. 519 (7543): 362–5. Bibcode:2015Natur.519..362D. doi:10.1038/nature13983. PMC 4369195. PMID 25533961.
- ^ Löwe J, Amos LA (January 1998). "Crystal structure of the bacterial cell-division protein FtsZ". Nature. 391 (6663): 203–6. Bibcode:1998Natur.391..203L. doi:10.1038/34472. PMID 9428770. S2CID 4330857.
- ^ "Digital Downloads". PurSolutions. Retrieved 2020-02-19.
- ^ a b Pilhofer M, Ladinsky MS, McDowall AW, Petroni G, Jensen GJ (December 2011). "Microtubules in bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton". PLOS Biology. 9 (12): e1001213. doi:10.1371/journal.pbio.1001213. PMC 3232192. PMID 22162949.
- ^ Williams RC, Shah C, Sackett D (November 1999). "Separation of tubulin isoforms by isoelectric focusing in immobilized pH gradient gels". Analytical Biochemistry. 275 (2): 265–7. doi:10.1006/abio.1999.4326. PMID 10552916.
- ^ "tubulin in Protein sequences". EMBL-EBI.
- ^ Heald R, Nogales E (January 2002). "Microtubule dynamics". Journal of Cell Science. 115 (Pt 1): 3–4. doi:10.1242/jcs.115.1.3. PMID 11801717.
- ^ Howard J, Hyman AA (April 2003). "Dynamics and mechanics of the microtubule plus end". Nature. 422 (6933): 753–8. Bibcode:2003Natur.422..753H. doi:10.1038/nature01600. PMID 12700769. S2CID 4427406.
- ^ a b Martin-Galiano AJ, Oliva MA, Sanz L, Bhattacharyya A, Serna M, Yebenes H, et al. (June 2011). "Bacterial tubulin distinct loop sequences and primitive assembly properties support its origin from a eukaryotic tubulin ancestor". The Journal of Biological Chemistry. 286 (22): 19789–803. doi:10.1074/jbc.M111.230094. PMC 3103357. PMID 21467045.
- ^ a b Schlieper D, Oliva MA, Andreu JM, Löwe J (June 2005). "Structure of bacterial tubulin BtubA/B: evidence for horizontal gene transfer". Proceedings of the National Academy of Sciences of the United States of America. 102 (26): 9170–5. Bibcode:2005PNAS..102.9170S. doi:10.1073/pnas.0502859102. PMC 1166614. PMID 15967998.
- ^ Deng X, Fink G, Bharat TA, He S, Kureisaite-Ciziene D, Löwe J (July 2017). "Prosthecobacter BtubAB show dynamic instability". Proceedings of the National Academy of Sciences of the United States of America. 114 (29): E5950 – E5958. doi:10.1073/pnas.1705062114. PMC 5530688. PMID 28673988.
- ^ Aylett CH, Wang Q, Michie KA, Amos LA, Löwe J (November 2010). "Filament structure of bacterial tubulin homologue TubZ". Proceedings of the National Academy of Sciences of the United States of America. 107 (46): 19766–71. Bibcode:2010PNAS..10719766A. doi:10.1073/pnas.1010176107. PMC 2993389. PMID 20974911.
- ^ Bharat TA, Murshudov GN, Sachse C, Löwe J (July 2015). "Structures of actin-like ParM filaments show architecture of plasmid-segregating spindles". Nature. 523 (7558): 106–10. Bibcode:2015Natur.523..106B. doi:10.1038/nature14356. PMC 4493928. PMID 25915019.
- ^ NCBI CCD cd2186
- ^ a b Zhou J, Giannakakou P (January 2005). "Targeting microtubules for cancer chemotherapy". Current Medicinal Chemistry. Anti-Cancer Agents. 5 (1): 65–71. doi:10.2174/1568011053352569. PMID 15720262.
- ^ "Mebendazole". Drugs.com. The American Society of Health-System Pharmacists. Archived from the original on December 11, 2019. Retrieved August 18, 2015.
- ^ "Albendazole". Drugs.com. The American Society of Health-System Pharmacists. Archived from the original on September 23, 2015. Retrieved August 18, 2015.
- ^ Serbus LR, Landmann F, Bray WM, White PM, Ruybal J, Lokey RS, et al. (September 2012). "A cell-based screen reveals that the albendazole metabolite, albendazole sulfone, targets Wolbachia". PLOS Pathogens. 8 (9): e1002922. doi:10.1371/journal.ppat.1002922. PMC 3447747. PMID 23028321.
- ^ Karki R, Mariani M, Andreoli M, He S, Scambia G, Shahabi S, Ferlini C (April 2013). "βIII-Tubulin: biomarker of taxane resistance or drug target?". Expert Opinion on Therapeutic Targets. 17 (4): 461–72. doi:10.1517/14728222.2013.766170. PMID 23379899. S2CID 26229777.
- ^ Ludueña RF (May 1993). "Are tubulin isotypes functionally significant". Molecular Biology of the Cell. 4 (5): 445–57. doi:10.1091/mbc.4.5.445. PMC 300949. PMID 8334301.
- ^ "TUBB1 tubulin, beta 1 class VI [Homo sapiens (human)]". Gene - NCBI.
- ^ a b Lecine P, et al. (August 2000). "Hematopoietic-specific beta 1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2". Blood. 96 (4): 1366–73. doi:10.1182/blood.V96.4.1366. PMID 10942379.
- ^ Yang H, Ganguly A, Yin S, Cabral F (March 2011). "Megakaryocyte lineage-specific class VI β-tubulin suppresses microtubule dynamics, fragments microtubules, and blocks cell division". Cytoskeleton. 68 (3): 175–87. doi:10.1002/cm.20503. PMC 3082363. PMID 21309084.
- ^ McNally FJ, Vale RD (November 1993). "Identification of katanin, an ATPase that severs and disassembles stable microtubules". Cell. 75 (3): 419–29. doi:10.1016/0092-8674(93)90377-3. PMID 8221885. S2CID 10264319.
- ^ Chumová J, Trögelová L, Kourová H, Volc J, Sulimenko V, Halada P, Kučera O, Benada O, Kuchařová A, Klebanovych A, Dráber P, Daniel G, Binarová P (2018). "γ-Tubulin has a conserved intrinsic property of self-polymerization into double stranded filaments and fibrillar networks". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1865 (5): 734–748. doi:10.1016/j.bbamcr.2018.02.009. PMID 29499229. S2CID 4053150.
- ^ Sontag CA, Sage H, Erickson HP (September 2009). "BtubA-BtubB heterodimer is an essential intermediate in protofilament assembly". PLOS ONE. 4 (9): e7253. Bibcode:2009PLoSO...4.7253S. doi:10.1371/journal.pone.0007253. PMC 2746283. PMID 19787042.
- ^ Margolin W (November 2005). "FtsZ and the division of prokaryotic cells and organelles". Nature Reviews. Molecular Cell Biology. 6 (11): 862–71. doi:10.1038/nrm1745. PMC 4757588. PMID 16227976.
- ^ Ni L, Xu W, Kumaraswami M, Schumacher MA (June 2010). "Plasmid protein TubR uses a distinct mode of HTH-DNA binding and recruits the prokaryotic tubulin homolog TubZ to effect DNA partition". Proceedings of the National Academy of Sciences of the United States of America. 107 (26): 11763–8. doi:10.1073/pnas.1003817107. PMC 2900659. PMID 20534443.
- ^ Chaikeeratisak, V; Nguyen, K; Egan, ME; Erb, ML; Vavilina, A; Pogliano, J (15 August 2017). "The Phage Nucleus and Tubulin Spindle Are Conserved among Large Pseudomonas Phages". Cell Reports. 20 (7): 1563–1571. doi:10.1016/j.celrep.2017.07.064. PMC 6028189. PMID 28813669.
- ^ Malone, Lucia M.; Warring, Suzanne L.; Jackson, Simon A.; Warnecke, Carolin; Gardner, Paul P.; Gumy, Laura F.; Fineran, Peter C. (9 December 2019). "A jumbo phage that forms a nucleus-like structure evades CRISPR–Cas DNA targeting but is vulnerable to type III RNA-based immunity". Nature Microbiology. 5 (1): 48–55. bioRxiv 10.1101/782524. doi:10.1038/s41564-019-0612-5. PMID 31819217. S2CID 209164667.
- ^ Akıl, Caner; Ali, Samson; Tran, Linh T.; Gaillard, Jeremie; Li, Wenfei; Hayashida, Kenichi; Hirose, Mika; Kato, Takayuki; Oshima, Atsunori; Fujishima, Kosuke; Blanchoin, Laurent; Narita, Akihiro; Robinson, Robert C. (2021). "Structure and dynamics of Odinarchaeota tubulin and the implications for eukaryotic microtubule evolution". bioRxiv 10.1101/2021.10.22.465531. doi:10.1101/2021.10.22.465531. S2CID 239831170.
{{cite journal}}: Cite journal requires|journal=(help) - ^ van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (March 2004). "The Catharanthus alkaloids: pharmacognosy and biotechnology". Current Medicinal Chemistry. 11 (5): 607–28. doi:10.2174/0929867043455846. PMID 15032608.
- ^ Raviña, Enrique (2011). "Vinca alkaloids". The evolution of drug discovery: From traditional medicines to modern drugs. John Wiley & Sons. pp. 157–159. ISBN 9783527326693.
- ^ Cooper, Raymond; Deakin, Jeffrey John (2016). "Africa's gift to the world". Botanical Miracles: Chemistry of Plants That Changed the World. CRC Press. pp. 46–51. ISBN 9781498704304.
- ^ Keglevich P, Hazai L, Kalaus G, Szántay C (May 2012). "Modifications on the basic skeletons of vinblastine and vincristine". Molecules. 17 (5): 5893–914. doi:10.3390/molecules17055893. PMC 6268133. PMID 22609781.
- ^ Ngo QA, Roussi F, Cormier A, Thoret S, Knossow M, Guénard D, Guéritte F (January 2009). "Synthesis and biological evaluation of vinca alkaloids and phomopsin hybrids". Journal of Medicinal Chemistry. 52 (1): 134–42. doi:10.1021/jm801064y. PMID 19072542.
- ^ Altmann, Karl-Heinz (2009). "Preclinical Pharmacology and Structure-Activity Studies of Epothilones". In Mulzer, Johann H. (ed.). The Epothilones: An Outstanding Family of Anti-Tumor Agents: From Soil to the Clinic. Springer Science & Business Media. pp. 157–220. ISBN 9783211782071.
- ^ Samson AL, Knaupp AS, Sashindranath M, Borg RJ, Au AE, Cops EJ, et al. (October 2012). "Nucleocytoplasmic coagulation: an injury-induced aggregation event that disulfide crosslinks proteins and facilitates their removal by plasmin". Cell Reports. 2 (4): 889–901. doi:10.1016/j.celrep.2012.08.026. PMID 23041318.
External links
[edit]- Tubulin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- EC 3.6.5.6
- Protocols for tubulin experiments
- High-resolution tubulin infographic
