Isotype (immunology)

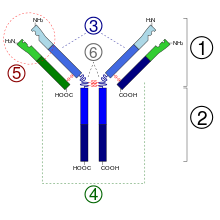
- Fab region
- Fc region
- Heavy chain (blue) with one variable (VH) domain followed by a constant domain (CH1), a hinge region, and two more constant (CH2 and CH3) domains
- Light chain (green) with one variable (VL) and one constant (CL) domain
- Antigen binding site (paratope)
- Hinge regions
In immunology, antibodies (immunoglobulins (Ig)) are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen (or more exactly, an epitope). In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen (triggering different elements of the innate immune system). They appear at different stages of an immune response, differ in structural features, and in their location around the body.[1]
Isotype expression reflects the maturation stage of a B cell. Naive B cells express IgM and IgD isotypes with unmutated variable genes, which are produced from the same initial transcript following alternative splicing. Expression of other antibody isotypes (in humans: IgG, IgA, and IgE) occurs via a process of class switching after antigen exposure.[2] Class switching is mediated by the enzyme AID (activation-induced cytidine deaminase) and occurs after the B cell binds an antigen through its B cell receptor. Class-switching usually requires interaction with a T helper cell.[3][4]
In humans, there are five heavy chain isotypes α,δ,γ,ε,μ, corresponding to five antibody isotypes:
- α – IgA, further divided into subclasses IgA1 and IgA2
- δ – IgD
- γ – IgG, further divided into subclasses IgG1 to IgG4
- ε – IgE
- μ – IgM
There are also two light chain isotypes κ and λ; however, there is no significant difference in function between the two. Thus an antibody isotype is determined by the constant regions of the heavy chains only.[1]
IgM is first expressed as a monomer on the surface of immature B cells. Upon antigenic stimulation, IgM+ B cells secrete pentameric IgM antibody formed by five Ig monomers which are linked via disulfide bonds. The pentamer also contains a polypeptide J-chain, which links two of the monomers and facilitates secretion at mucosal surfaces. The pentameric structure of IgM antibodies makes them efficient at binding antigens with repetitive epitopes (e.g. bacterial capsule, viral capsid) and activation of complement cascade. As IgM antibodies are expressed early in a B cell response, they are rarely highly mutated and have broad antigen reactivity thus providing an early response to a wide range of antigens without the need for T cell help.[5]
IgD isotypes are expressed on naive B cells as they leave bone marrow and populate secondary lymphoid organs. The levels of surface expression of IgD isotype has been associated with differences in B cell activation status but their role in serum is poorly understood.[6]
The IgG, IgE and IgA antibody isotypes are generated following class-switching during germinal centre reaction and provide different effector functions in response to specific antigens. IgG is the most abundant antibody class in the serum and it is divided into 4 subclasses based on differences in the structure of the constant region genes and the ability to trigger different effector functions. Despite the high sequence similarity (90% identical on the amino acid level), each subclass has a different half-life, a unique profile of antigen binding and distinct capacity for complement activation. IgG1 antibodies are the most abundant IgG class and dominate the responses to protein antigens. Impaired production of IgG1 is observed in some cases of immunodeficiency and is associated with recurrent infections.[7] The IgG responses to bacterial capsular polysaccharide antigens are mediated primarily via IgG2 subclass, and deficiencies in this subclass result in susceptibility to certain bacterial species.[8] IgG2 represents the major antibody subclass reacting to glycan antigens but IgG1 and IgG3 subclasses have also been observed in such responses, particularly in the case of protein-glycan conjugates.[9]
IgG3 is an efficient activator of pro-inflammatory responses by triggering the classical complement pathway.[10] It has the shortest half-life compared to the other IgG subclasses[11] and is frequently present together with IgG1 in response to protein antigens after viral infections.[12] IgG4 is the least abundant IgG subclass in the serum and is often generated following repeated exposure to the same antigen or during persistent infections.
IgA antibodies are secreted in the respiratory or the intestinal tract and act as the main mediators of mucosal immunity.[13] They are monomeric in the serum, but appear as a dimer termed secretory IgA (sIgA) at mucosal surfaces. The secretory IgA is associated with a J-chain and another polypeptide chain called the secretory component.[14] IgA antibodies are divided into two subclasses that differ in the size of their hinge region.[15] IgA1 has a longer hinge region which increases its sensitivity to bacterial proteases.[16] Therefore, this subclass dominates the serum IgA, while IgA2 is predominantly found in mucosal secretions. Complement fixation by IgA is not a major effector mechanism at the mucosal surface but IgA receptor is expressed on neutrophils which may be activated to mediate antibody-dependent cellular cytotoxicity.[17] sIgA has also been shown to potentiate the immune response in intestinal tissue by uptake of antigen together with the bound antibody by dendritic cells.[18]
IgE antibodies are present at lowest concentrations in peripheral blood but constitute the main antibody class in allergic responses through the engagement of mast cells, eosinophils and Langerhans cells.[19] Responses to specific helminths are also characterised with elevated levels of IgE antibodies.[20]
See also
[edit]References
[edit]- ^ a b Janeway, CA; Travers, P; Walport, M; et al. (2001). "Immunobiology: The Immune System in Health and Disease. 5th edition". NCBI. NCBI. Retrieved 2016-01-19.
- ^ Stavnezer, Janet (1996). "Immunoglobulin Class Switching". Current Opinion in Immunology. 8 (2): 199–205. doi:10.1016/s0952-7915(96)80058-6. PMID 8725943.
- ^ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002-01-01). "Helper T Cells and Lymphocyte Activation".
- ^ Chandra, Vivek; Bortnick, Alexandra; Murre, Cornelis (2015-09-01). "AID targeting: old mysteries and new challenges". Trends in Immunology. 36 (9): 527–535. doi:10.1016/j.it.2015.07.003. PMC 4567449. PMID 26254147.
- ^ Chen, J, Boes, M (1998). "A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection". J Exp Med. 188 (12): 2381–6. doi:10.1084/jem.188.12.2381. PMC 2212438. PMID 9858525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Achatz, G., Geisberger, R. (2006). "The riddle of the dual expression of IgM and IgD". Immunology. 118 (4): 429–37. doi:10.1111/j.1365-2567.2006.02386.x. PMC 1782314. PMID 16895553.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Acton, R. T., Barton, J. C. (2016). "Selective Subnormal IgG1 in 54 Adult Index Patients with Frequent or Severe Bacterial Respiratory Tract Infections". J Immunol Res. 2016: 1405950. doi:10.1155/2016/1405950. PMC 4830719. PMID 27123464.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Out, T. A., Kuijpers, T. W. (1992). "IgG subclass deficiencies and recurrent pyogenic infections, unresponsiveness against bacterial polysaccharide antigens". Allergol Immunopathol (Madr). 20 (1): 28–34. PMID 1509985.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jonsdottir, I., Vidarsson, G. (1998). "Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine". Infect Immun. 66 (6): 2866–70. doi:10.1128/IAI.66.6.2866-2870.1998. PMC 108283. PMID 9596761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barandun, S., Morell, A. (1972). "IgG subclasses: development of the serum concentrations in "normal" infants and children". J Pediatr. 80 (6): 960–4. doi:10.1016/s0022-3476(72)80007-6. PMID 4623683.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith, C. I., Hassan, M. S. (1991). "Biological half-life of normal and truncated human IgG3 in scid mice". Eur J Immunol. 21 (5): 1319–22. doi:10.1002/eji.1830210534. PMID 2037016. S2CID 45417913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wahren, B., Linde, G. A. (1983). "Virus-specific antibody activity of different subclasses of immunoglobulins G and A in cytomegalovirus infections". Infect Immun. 42 (1): 237–44. doi:10.1128/iai.42.1.237-244.1983. PMC 264549. PMID 6311746.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Honjo, T.; Făgărășan, Sidonia (2001). "In situ class switching and differentiation to IgA-producing cells in the gut lamina propria". Nature. 413 (6856): 639–43. Bibcode:2001Natur.413..639F. doi:10.1038/35098100. PMID 11675788. S2CID 4393498.
- ^ Hilschmann, N., Bastian, A. (1992). "Intra- and interchain disulfide bridges of the human J chain in secretory immunoglobulin A". Biol Chem Hoppe-Seyler. 373 (2): 1255–63. doi:10.1515/bchm3.1992.373.2.1255. PMID 1292512.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Perkins, S. J., Furtado, P. B. (2004). "Solution structure determination of monomeric human IgA2 by X-ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1". J Mol Biol. 338 (5): 921–41. doi:10.1016/j.jmb.2004.03.007. PMID 15111057.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Frandsen, E. V., Kilian, M. (1992). "Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence". APMIS. 104 (1–6): 321–38. doi:10.1111/j.1699-0463.1996.tb00724.x. PMID 8703438. S2CID 19432279.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Palese, P., Mullarkey, C. E. (2016). "Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner". mBio. 7 (5): e01624-16. doi:10.1128/mBio.01624-16. PMC 5050345. PMID 27703076.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ van Kooten, C., Heystek, H. C. (2002). "Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation". J Immunol. 168 (1): 102–7. doi:10.4049/jimmunol.168.1.102. PMID 11751952.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Walport M, Janeway CA Jr (2001). Immunobiology: The Immune System in Health and Disease.
- ^ Groenen, P. J, Appenzeller, S. (2015). "Immunoglobulin rearrangement analysis from multiple lesions in the same patient using next-generation sequencing". Histopathology. 67 (6): 843–58. doi:10.1111/his.12714. PMID 25891511. S2CID 36669923.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
[edit]- Immunoglobulin+Isotypes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview at University of South Carolina School of Medicine
- Overview at Southern Illinois University Carbondale
