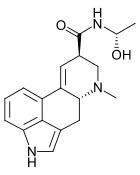
Lysergic acid hydroxyethylamide
 | |
| Clinical data | |
|---|---|
| Other names | ᴅ-lysergic acid methyl carbinolamide |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.079 |
| Chemical and physical data | |
| Formula | C18H21N3O2 |
| Molar mass | 311.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
ᴅ-Lysergic acid α-hydroxyethylamide (LAH, LSH), also known as ᴅ-lysergic acid methyl carbinolamide, is an ergoamide and an ergoline. It is perhaps the main constituent of the parasitic fungus, Claviceps paspali;[2][3][4] and found in trace amounts in Claviceps Purpurea.[5][6] C. paspali and C. purpurea are ergot-spreading fungi. Periglandula, Clavicipitacepus fungi, are permanently symbiotically connected to an estimated 450 species of Convolvulaceae[7] and thus generate LAH in some of them (42 generate ergolines, by Eckart Eich's review[8]). The most well-known ones are Ipomoea tricolor (“morning glory”), Turbina corymbosa (coaxihuitl), and Argyreia nervosa (Hawaiian baby woodrose).
The more well-known analog, lysergic acid amide (syn. ergine), is more prominent in analytical results because LAH easily decomposes to ergine.[9][10] Ergine is only present because of the decomposition of LAH (and lysergic acid hydroxymethylethylamide and ergopeptines or their ergopeptam precursors); it is not generated.[11][12][13][14][15] Indeed, a 2016 analysis found that fresher I. tricolor seeds contained more LAH than the other two batches analyzed[16] (another interesting finding is that penniclavine was the predominant ergoline.)
Chemistry
[edit]The structure is similar to LSD, with the N,N- diethylamide group replaced by an N- (1- hydroxyethyl)amide in D-lysergic acid α-hydroxyethylamide.
Pharmacology
[edit]LSH has an affinity for several dopamine and serotonin receptors, mainly the 5-HT2 receptor like most psychedelics in the human brain. LSD is a partial agonist on many dopamine and serotonin receptors, so it is highly likely that LSH is also an agonist at dopamine and serotonin receptors.
[...] the new naturally occurring alkaloid ᴅ-lysergic acid methyl carbinolamide has powerful ergometrine-like oxytocic action and weak ergotamine-like adrenergic blocking actions. It must be included, on the basis of pharmacological evidence, in the ergometrine group of ergot alkaloids. Ergometrine, however, is less toxic and more active than the new alkaloid. Results suggest that it could have a lysergic acid diethylamide-like activity, but this hypothesis must be checked by experiments on humans.[17]
— Glasser, A.
Legality
[edit]ᴅ-lysergic acid α-hydroxyethylamide is unscheduled and uncontrolled in the United States, but possession and sales of it for human consumption could potentially be prosecuted under the Federal Analog Act because of its structural similarities to LSD. Although doubtful as it breaks down into LSA which is a Schedule 3 drug and therefore not applicable to the Federal Analog Act.
See also
[edit]- Ergoline
- Lysergic acid
- LSA
- LSD
- Ergot
- Hawaiian baby woodrose (Argyreia nervosa)
- Ololiuhqui (Rivea corymbosa)
- Tlitliltzin (Ipomoea tricolor)[verification needed][citation needed]
References
[edit]- ^ "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- ^ Arcamone F, Bonino C, Chain EB, Ferretti A, Pennella P, Tonolo A, Vero L (July 1960). "Production of lysergic acid derivatives by a strain of Claviceps paspali Stevens and Hall in submerged culture". Nature. 187 (4733): 238–239. Bibcode:1960Natur.187..238A. doi:10.1038/187238a0. PMID 13794048.
- ^ Castagnoli N, Corbett K, Chain EB, Thomas R (April 1970). "Biosynthesis of N-(alpha-hydroxyethyl) lysergamide, a metabolite of Claviceps paspali Stevens and Hall". The Biochemical Journal. 117 (3) (published 1970-04-01): 451–455. doi:10.1042/bj1170451. PMC 1178946. PMID 5419742.
- ^ Basmadjian G, Floss HG, Gröger D, Erge D (1969). "Biosynthesis of ergot alkaloids. Lysergylalanine as precursor of amide-type alkaloids". J. Chem. Soc. D (8): 418–419. doi:10.1039/C29690000418. ISSN 0577-6171.
- ^ Schultes R (1973). "4. Plants of Hallucinogenic Use / The Fungi". The Botany and Chemistry of Hallucinogens. Springfield, IL: Charles Thomas. p. 37. ISBN 9780398064167.
"Whereas ergine, lysergic acid hydroxyethylamide, and lysergyl L-valine methylester occur in ergot of rye only in trace amounts, ergonovine (synonyms ergometrine, ergobasin), which is the specific oxytocic factor of a ergot, is often found in remarkable quantities. In contrast, ergine and hydroxyethylamide of lysergic acid are the main constituents of certain ergot growing on wild grasses, e.g. Paspalum distichum." 4. Plants of Hallucinogenic Use / The Fungi, p. 37 - ^ Wasson RG, Hofmann A, Ruck CA, Webster P (November 25, 2008) [1978]. Forte R (ed.). The Road to Eleusis: Unveiling the Secret of the Mysteries (30th Anniversary ed.). Berkeley, Calif.: North Atlantic Books. ISBN 978-1-55643-752-6.
"We analyzed ergot of wheat and ergot of barley in our laboratory and they were found to contain basically the same alkaloids as ergot of rye, viz. alkaloids of the ergotamine and ergotoxine group, ergonovine, and sometimes also traces of lysergic acid amide. As I said before, ergonovine and lysergic acid amide, both psychoactive, are soluble in water whereas the other alkaloids are not." Albert Hofmann, 2. A Challenging Question and my Answer, p. 42 - ^ Leistner E, Steiner U (February 3, 2018). "The Genus Periglandula and Its Symbiotum with Morning Glory Plants (Convolvulaceae)". In Anke T, Schüffler A (eds.). Physiology and Genetics. Cham: Springer International Publishing. pp. 131–147. doi:10.1007/978-3-319-71740-1_5. ISBN 978-3-319-71739-5. Retrieved 2024-11-21.
- ^ Eich E (January 12, 2008). "4.2 Ergolines". Solanaceae and convolvulaceae - secondary metabolites: biosynthesis, chemotaxonomy, biological and economic significance: a handbook. Berlin, Heidelberg: Springer-Verlag. doi:10.1007/978-3-540-74541-9. ISBN 978-3-540-74540-2. OCLC 195613136
Table 4.1 Unambiguously ergoline-positive Ipomoea species (pages 225-227)
Table 4.4 Unambiguously ergoline-positive Argyreia species (p. 236)
Table 4.5 Unambiguously ergoline-positive Stictocardia and Turbina species (p. 238){{cite book}}: CS1 maint: postscript (link) - ^ Shulgin A (2012-12-02) [1976]. "4. Psychotomimetic Agents". In Maxwell G (ed.). Psychopharmacological agents. Medicinal Chemistry. Vol. 4. New York: Academic Press. pp. 71–72. ISBN 978-0-12-290559-9.
"These compounds, although well documented as components in the Convolvulaceae, are possibly lost in several of the analyses of alkaloid composition. They are extremely unstable, and are very readily degraded into acetaldehyde and the corresponding amide, ergine or isoergine." (p. 72) - ^ Schultes RE, Hofmann A (1973). The Botany and Chemistry of Hallucinogens. Springfield, IL: Charles Thomas. p. 246. ISBN 9780398064167.
"Later, it was found that ergine and isoergine were present in the seeds to some extent in the form of lysergic acid N-(1-hydroxyethyl) amide and isolysergic acid N-(1-hydroxyethyl) amide, respectively, and that, during the isolation procedure, they easily hydrolize to ergine and isoergine, respectively, and acetaldehyde." 4. Plants of Hallucinogenic Use / Convolvulaceae, p. 246 - ^ Flieger M, Sedmera P, Vokoun J, R̆ic̄icovā A, R̆ehác̆ek Z (1982-02-19). "Separation of four isomers of lysergic acid α-hydroxyethylamide by liquid chromatography and their spectroscopic identification". Journal of Chromatography A. 236 (2): 441–452. doi:10.1016/S0021-9673(00)84895-5. ISSN 0021-9673.
- ^ Ramstad E (1968). "Chemistry of alkaloid formation in ergot". Lloydia. 31: 327–341.
- ^ Kleinerová E, Kybal J (September 1973). "Ergot alkaloids. IV. Contribution to the biosynthesis of lysergic acid amides". Folia Microbiologica. 18 (5): 390–392. doi:10.1007/BF02875934. PMID 4757982.
- ^ Panaccione DG, Tapper BA, Lane GA, Davies E, Fraser K (October 2003). "Biochemical outcome of blocking the ergot alkaloid pathway of a grass endophyte". Journal of Agricultural and Food Chemistry. 51 (22): 6429–6437. doi:10.1021/jf0346859. PMID 14558758.
- ^ Panaccione DG (2010). "Ergot alkaloids". In Hofrichter M (ed.). The Mycota, Industrial Applications. Vol. 10 (2nd ed.). Berlin-Heidelburg, Germany: Springer-Verlag. pp. 195–214.
- ^ Nowak J, Woźniakiewicz M, Klepacki P, Sowa A, Kościelniak P (May 2016). "Identification and determination of ergot alkaloids in Morning Glory cultivars". Analytical and Bioanalytical Chemistry. 408 (12) (published February 14, 2016): 3093–3102. doi:10.1007/s00216-016-9322-5. PMC 4830885. PMID 26873205.
"As has been demonstrated in this study, LSH is a labile compound, and therefore the variances in its concentration may be due to different age and storage conditions of the seeds rather than difference in plant metabolism. Indeed, seeds IT-HB2, which express highest concentration of LSH, were bought directly from the producer, whereas seeds IP-HB1 were purchased in retail stores." (Analysis of different Ipomoea seeds)
See Table 3 under "Analysis of different Ipomoea seeds".
Concentration values for "LSH", "Lyzergol/isobars", penniclavine, and chanoclavine can be obtained by dividing the concentration values of ergine or ergometrine by their relative abundance values and multiplying that number by the relative abundance value of the specified chemical. - ^ Glasser A (January 1961). "Some pharmacological actions of ᴅ-lysergic acid methyl carbinolamide". Nature. 189 (4761): 313–4. Bibcode:1961Natur.189..313G. doi:10.1038/189313a0. PMID 13705953. S2CID 4260358.
External links
[edit]- Ergot - A Rich Source of Pharmacologically Active Substances by Albert Hofmann
