2023 in paleontology
.
| |||
|---|---|---|---|
Paleontology or palaeontology is the study of prehistoric life forms on Earth through the examination of plant and animal fossils.[1] This includes the study of body fossils, tracks (ichnites), burrows, cast-off parts, fossilised feces (coprolites), palynomorphs and chemical residues. Because humans have encountered fossils for millennia, paleontology has a long history both before and after becoming formalized as a science. This article records significant discoveries and events related to paleontology that occurred or were published in the year 2023.
| 2023 in science |
|---|
| Fields |
| Technology |
| Social sciences |
| Paleontology |
| Extraterrestrial environment |
| Terrestrial environment |
| Other/related |
Flora
[edit]"Algae"
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Country | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Qin et al. |
Late Paleoproterozoic[3] to Tonian |
Liulaobei Formation |
|||||
|
Glossophyton shouxianensis[2] |
Sp. nov |
Qin et al. |
Tonian |
Liulaobei Formation |
||||
|
Gen. et sp. nov |
Valid |
Liu et al. |
Ediacaran |
A possible brown alga. The type species is H. yuxiensis. Announced in 2023; the final version of the article naming it was published in 2024. |
||||
|
Sp. nov |
Qin et al. |
Late Paleoproterozoic[3] to Tonian |
Liulaobei Formation |
Phycological research
[edit]- Liu et al. (2023) report the discovery of approximately 1.64-billion-years-old multicellular fossils from the Chuanlinggou Formation (China), providing evidence that the multicellular algae had already originated by the late Paleoproterozoic.[3]
- A study on deep-sea organic-rich deposits from the Waiheke Island (New Zealand) is published by Grasby et al. (2023), who interpret the studied deposits as lamalginites formed from phytoplankton, providing evidence of an open-ocean algal bloom during the Permian-Triassic transition.[5]
- A study on the fossil material of rhodolith-forming coralline red algae from the Miocene Long Formation from the Little Andaman (India), interpreted as indicative of high carbonate production in the northeastern Indian Ocean during the Serravallian, is published by Dey et al. (2023).[6]
Plants
[edit]Fungi
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Valid |
Kundu & Khan |
Miocene |
A member of the family Meliolaceae. Announced in 2023; the final version of the article naming it was published in 2024. |
||||
|
Gen. et sp. nov |
A lichen-like thalli. |
|||||||
|
Gen. et sp. nov |
Strullu-Derrien & Hawksworth in Strullu-Derrien et al. |
Devonian |
A member of Ascomycota of uncertain affinities. |
|||||
|
Gen. et sp. nov |
Krings & Harper |
Devonian |
A fungal mycelium of uncertain affinities. Genus includes new species R. endoconidiarum. |
|||||
|
Gen. et sp. nov |
Worobiec & Piątek in Worobiec, Piątek & Worobiec |
Pliocene |
A member of Ascomycota of uncertain affinities, with resemblances to modern powdery mildews. The type species is S. pliocenicus. |
Mycological research
[edit]- New information on the anatomy of Sporocarpon asteroides, inferred from new specimens from the Lower Coal Measures (United Kingdom), is presented by Krings et al. (2023), who interpret S. asteroides as a unisporic sporocarp with a parenchyma-like peridium, probably with affinities to the Glomeromycota.[12]
- The first finding of a mycelium of a putative nematophagous fungus belonging to the group Basidiomycota from the Cretaceous of North Asia is reported from the Santonian Yantardakh Lagerstätte (Taymyr, Russia) by Sukhomlyn & Perkovsky (2023).[13]
- Kutchiathyrites eccentricus is transferred to the modern genus Mycoenterolobium by Worobiec, Worobiec & Liu (2023) on the basis of study of the fossil material from the Neogene deposits of the Gray Fossil Site (Tennessee, United States) and the Bełchatów Lignite Mine (Poland).[14]
General floral research
[edit]Cnidarians
[edit]New cnidarian taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Country | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Valid |
Niko & Suzuki |
Miocene |
Kennon Limestone |
A species of Acropora. |
|||
|
Sp. nov |
El-Desouky, Herbig & Kora |
Carboniferous (Kasimovian) |
Aheimer Formation |
A rugose coral belonging to the group Stauriida and the family Antiphyllidae. |
||||
|
Sp. nov |
Valid |
Samaniego-Pesqueira, Löser & Moreno-Bedmar |
Early Cretaceous (Albian) |
A coral belonging to the superfamily Stylinoidea and the family Aulastraeoporidae. |
||||
|
Sp. nov |
Valid |
Samaniego-Pesqueira, Löser & Moreno-Bedmar |
Early Cretaceous (Albian) |
Espinazo del Diablo Formation |
A coral belonging to the superfamily Stylinoidea and the family Aulastraeoporidae. |
|||
|
Gen. et sp. nov |
Valid |
Larson & Briggs |
Silurian (Přídolí) |
A hydrozoan belonging to the group Capitata and the superfamily Porpitoidea. The type species is B. ciurcae. |
||||
|
Gen. et sp. nov |
Valid |
Moon, Caron & Moysiuk |
Cambrian |
A medusozoan, possibly a member of the stem group to Cubozoa or Acraspeda; described on the basis of fossil material representing a free-swimming medusa. The type species is B. phasmiformis. |
||||
|
Sp. nov |
Valid |
Niko |
Okanaro Group |
A tabulate coral belonging to the group Halysitida and the family Halysitidae. |
||||
|
Sp. nov |
Song et al. |
Cambrian (Fortunian) |
Kuanchuanpu Formation |
A medusozoan, possibly a member of Conulata. |
||||
|
Sp. nov |
Valid |
Löser, Werner & Darga |
Late Cretaceous (Cenomanian) |
A coral belonging to the superfamily Stylinoidea and the family Stylinidae. |
||||
|
Gen. et sp. nov |
Valid |
Niko |
Devonian |
Naidaijin Formation |
A coral belonging to the group Auloporida and the family Bajgoliidae. The type species is K. patulus. |
|||
|
Sp. nov |
El-Desouky, Herbig & Kora |
Carboniferous (Kasimovian) |
Aheimer Formation |
A rugose coral belonging to the group Stauriida and the family Antiphyllidae. |
||||
|
Lytvolasma paraaucta[16] |
Sp. nov |
El-Desouky, Herbig & Kora |
Carboniferous (Kasimovian) |
Aheimer Formation |
A rugose coral belonging to the group Stauriida and the family Antiphyllidae. |
|||
|
Sp. nov |
El-Desouky, Herbig & Kora |
Carboniferous (Kasimovian) |
Aheimer Formation |
A rugose coral belonging to the group Stauriida and the family Antiphyllidae. |
||||
|
Sp. nov |
Valid |
Niko |
Miocene |
Katsuta Group |
A stony coral. |
|||
|
Gen. et comb. nov |
Qu, Li & Ou |
Cambrian |
A stem-medusozoan; a new genus for "Burithes" yunnanensis Hou et al. (1999). |
|||||
|
Gen et sp. nov |
Mergl & Kraft |
Devonian (Emsian) |
Zlíchov Formation |
A member of Scyphozoa belonging to the group Byroniida and the family Byroniidae. The type species is P. elegans. |
||||
|
Gen. et sp. nov |
Valid |
Samaniego-Pesqueira, Löser & Moreno-Bedmar |
Early Cretaceous (Albian) |
Espinazo del Diablo Formation |
A coral belonging to the superfamily Eugyroidea and the family Solenocoeniidae. The type species is P. sonorensis. |
|||
|
Gen. et sp. nov |
Mergl & Kraft |
Devonian (Emsian) |
Zlíchov Formation |
A member of Conulariida. The type species is P. tubulata. |
||||
|
Sp. nov |
Mergl & Kraft |
Devonian (Lochkovian) |
Lochkov Formation |
A member of Scyphozoa belonging to the group Byroniida and the family Byroniidae. |
||||
|
Prestephanoscyphus robustus[26] |
Sp. nov |
Mergl & Kraft |
Devonian (Eifelian) |
Srbsko Formation |
A member of Scyphozoa belonging to the group Byroniida and the family Byroniidae. |
|||
|
Sp. nov |
Lathuilière et al. |
Middle Jurassic (Bajocian) |
A coral. |
|||||
|
Sp. nov |
Valid |
Guedes, Siviero & Scheffler |
Devonian (Pragian–early Emsian) |
Ponta Grossa Formation |
A member of Conulariida. |
|||
|
Sp. nov |
Hachour et al. |
Neoproterozoic |
Cheikhia-Bir Amrane Group |
A scyphozoan of uncertain affinities. |
||||
|
Sp. nov |
Valid |
Elias & Hewitt |
Ordovician (Hirnantian) |
Whirlpool Formation |
A rugose coral. |
|||
|
Sp. nov |
Valid |
Niko |
Early Cretaceous (Aptian) |
Miyako Group |
A stony coral, a species of Stylina. |
|||
|
Gen. et sp. nov |
Valid |
Reich & Kutscher |
Silurian |
Hemse Group |
An octocoral belonging to the group Malacalcyonacea. The type species is S. leipnitzae. |
Cnidarian research
[edit]- Pywackia baileyi, originally classified as a bryozoan, is reinterpreted as a cnidarian by Hageman & Vinn (2023).[33]
- Conulariid specimens preserved with muscle bundles and a possible gastric cavity are described from the Carboniferous Wewoka and Graham formations (Oklahoma and Texas, United States) by Sendino et al. (2023).[34]
- Van Iten et al. (2023) describe soft parts of two specimens of Metaconularia manni from the Silurian (Sheinwoodian) Scotch Grove Formation (Iowa, United States), and interpret their anatomy as indicating that at least one species of conulariid might have lacked a free-living, medusoid life phase, and might have produced eggs and sperm within the body of the sessile polyp.[35]
- Redescription of Conicula striata is published by Zhao et al. (2023), who report that C. striata had features of both anthozoans and medusozoan polyps, and recover it as a stem-medusozoan, potentially indicating that medusozoans had an anemone-like ancestor.[36]
- Zhang et al. (2023) describe new fossil material of Qinscyphus necopinus from the Cambrian (Fortunian) Kuanchuanpu Formation (China), including the whole apical part and providing complete information on the morphology of Qinscyphus.[37]
- Plotnick, Young & Hagadorn (2023) classify Essexella asherae as a sea anemone, and reinterpret Reticulomedusa greenei as the pedal or oral disc of E. asherae.[38]
Arthropods
[edit]Bryozoans
[edit]New bryozoan taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Valid |
Ernst & Rodríguez |
Devonian (Emsian) |
A trepostome bryozoan belonging to the family Anisotrypidae. |
||||
|
Gen. et sp. nov |
Valid |
Ernst & Rodríguez |
Devonian (Pragian) |
A trepostome bryozoan of uncertain affinities. The type species is C. tenuis. |
||||
|
Gen. et sp. nov |
Valid |
Ernst & Rodríguez |
Devonian (Pragian–Emsian) |
A cyclostome bryozoan belonging to the family Diploclemidae. The type species is D. serenensis. |
||||
|
Sp. nov |
Valid |
Koromyslova |
Early Cretaceous (probably Hauterivian) |
A cyclostome bryozoan belonging to the family Eleidae. |
||||
|
Sp. nov |
Valid |
Arakawa |
Miocene (Langhian) |
A member of the family Phidoloporidae. Published online in 2022, but the issue date is listed as January 2023.[41] |
||||
|
Sp. nov |
Valid |
Ernst & Rodríguez |
Devonian (Pragian) |
A trepostome bryozoan belonging to the family Atactotoechidae. |
||||
|
Leptotrypa parva[39] |
Sp. nov |
Valid |
Ernst & Rodríguez |
Devonian (Emsian) |
A trepostome bryozoan belonging to the family Atactotoechidae. |
|||
|
Sp. nov |
Valid |
López-Gappa & Pérez |
Miocene |
Monte León Formation |
A member of the family Cellariidae. |
|||
|
Sp. nov |
In press |
Arakawa |
Pleistocene |
A species of Microporina. |
||||
|
Sp. nov |
In press |
Arakawa |
Pleistocene |
Setana Formation |
A species of Microporina. |
|||
|
Sp. nov |
In press |
Arakawa |
Pleistocene |
Setana Formation |
A species of Microporina. |
|||
|
Sp. nov |
In press |
Arakawa |
Pleistocene |
Setana Formation |
A species of Microporina. |
|||
|
Sp. nov |
Valid |
Tolokonnikova & Fedorov |
Ordovician (Sandbian) |
A cryptostome bryozoan. |
||||
|
Sp. nov |
Valid |
Koromyslova |
Early Cretaceous (probably Hauterivian) |
A cyclostome bryozoan. |
||||
|
Gen. et sp. nov |
Valid |
Ernst & Rodríguez |
Devonian (Pragian–Emsian) |
A cryptostome bryozoan belonging to the group Rhabdomesina. The type species is S. dubia. |
||||
|
Nom. nov |
Valid |
Pacaud |
Miocene |
A member of Cyclostomata belonging to the family Spiroporidae; a replacement name for Spiropora elegans Millet de la Turtaudière (1865). |
||||
|
Gen. et sp. nov |
Ernst & Tolokonnikova |
Ordovician |
A possible cystoporate bryozoan. The type species is S. ebbestadi. |
|||||
|
Gen. et sp. nov |
Valid |
Ernst |
Ordovician (Sandbian) |
Viivikonna Formation |
A trepostome bryozoan belonging to the family Monticuliporidae. The type species is T. kohtlaensis. |
Brachiopods
[edit]New brachiopod taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
In press |
Wang et al. |
Ordovician |
Huadan Formation |
||||
|
Sp. nov |
Valid |
Mergl & Šmídtová |
Devonian (Pragian) |
Vinařice Limestone |
A member of the family Discinidae. |
|||
|
Gen. et sp. nov |
Valid |
Serobyan et al. |
Devonian (Frasnian, possibly also Famennian) |
A member of Spiriferida belonging to the family Cyrtospiriferidae. The type species is A. arakelyani; genus might also include "Cyrtospirifer" kursaensis Sidjachenko (1962) and "Cyrtospirifer" limatus Solkina in Sidjachenko (1962) . |
||||
|
Sp. nov |
Valid |
Bitner et al. |
Oligocene (Rupelian) |
Lower Red Formation |
A species of Argyrotheca. |
|||
|
Gen. et sp. nov |
Valid |
Radulović et al. |
Jurassic |
Genus includes new species B. ponorensis. |
||||
|
Sp. nov |
Valid |
Radulović et al. |
Jurassic |
|||||
|
Sp. nov |
Valid |
Lavié & Benedetto |
Ordovician (Tremadocian) |
Pupusa Formation |
A member of Siphonotretida belonging to the family Siphonotretidae. |
|||
|
Sp. nov |
Valid |
MacFarlan |
Early Jurassic |
A member of Spiriferinida belonging to the group Paralaballidae. |
||||
|
Sp. nov |
Valid |
Baranov et al. |
Devonian (Famennian) |
Khoshyeilagh Formation |
A member of Athyridida. |
|||
|
Sp. nov |
Baeza-Carratalá, Berrocal-Casero & García Joral |
Early Cretaceous (Albian) |
Represa Formation |
|||||
|
Cyclothyris ementitum[57] |
Sp. nov |
Valid |
Berrocal-Casero, Baeza-Carratalá & García Joral |
Cretaceous (Albian–Cenomanian) |
Represa Formation |
|||
|
Sp. nov |
Valid |
Pérez et al. |
Miocene |
A species of Discinisca. |
||||
|
Sp. nov |
Valid |
Pérez et al. |
Miocene |
Gaiman Formation |
A species of Discinisca. |
|||
|
Sp. nov |
Zhang, Zhang & Holmer in Zhang et al. |
Cambrian Series 2 |
Shuijingtuo Formation |
A member of Linguloidea belonging to the family Eoobolidae. |
||||
|
Ssp. nov |
In press |
Harper & Bates |
Ordovician (Dapingian) |
Tagoat Group |
A plectorthid brachiopod. |
|||
|
Sp. nov |
Valid |
Waterhouse |
Permian (Asselian) |
Rammutt Formation |
A member of the family Rugosochonetidae. |
|||
|
Gen. et comb. nov |
Valid |
Tazawa & Waterhouse |
Permian |
Kanokura Formation |
A member of Productida belonging to the superfamily Paucispiniferoidea and the family Anidanthidae. The type species is "Terrakea" japonica Tazawa (2008). |
|||
|
Sp. nov |
Wu et al. |
Early Triassic (Olenekian) |
Nanpanjiang Basin |
A member of Spiriferinida belonging to the family Bittnerulidae. |
||||
|
Gen. et 2 sp. nov |
Valid |
Oh et al. |
Ordovician (Darriwilian) |
Jigunsan Formation |
A member of Strophomenoidea belonging to the family Rafinesquinidae. The type species is J. guraeriensis; genus also includes J. hambaeksanensis. |
|||
|
Sp. nov |
In press |
Wang et al. |
Ordovician |
Huadan Formation |
||||
|
Gen. et sp. nov |
Zhang, Zhang & Holmer in Zhang et al. |
Cambrian Series 2 |
Shuijingtuo Formation |
A member of Linguloidea belonging to the family Eoobolidae. The type species is L. xiaoyangbaensis. |
||||
|
Gen. et sp. nov |
Valid |
Baliński in Baliński & Halamski |
Devonian (Givetian) |
Skały Formation |
A member of Spiriferida belonging to the family Cyrtiidae. The type species is L. rara. |
|||
|
Sp. nov |
Valid |
MacFarlan |
Late Triassic (Rhaetian) |
A member of Terebratulida belonging to the family Lobothyrididae. |
||||
|
Sp. nov |
Valid |
Baranov et al. |
Devonian (Famennian) |
Khoshyeilagh Formation |
A member of Spiriferida. |
|||
|
Sp. nov |
Valid |
Baliński & Halamski |
Devonian (Givetian) |
Skały Formation |
A member of Spiriferida belonging to the family Ambocoeliidae. |
|||
|
Gen. et sp. nov |
In press |
Wang et al. |
Ordovician |
Huadan Formation |
Genus includes new species N. longisepta. |
|||
|
Sp. nov |
Valid |
Waterhouse |
Permian |
A member of Strophomenata belonging to the superfamily Orthotetoidea and the family Schuchertellidae. |
||||
|
Gen. et comb. nov |
In press |
Harper & Bates |
Ordovician |
An alimbellid brachiopod. The type species is "Orthis" bailyana Davidson. |
||||
|
Sp. nov |
Wu et al. |
Early Triassic (Olenekian) |
Nanpanjiang Basin |
A member of Terebratulida belonging to the family Angustothyrididae. |
||||
|
Ssp. nov |
Valid |
Waterhouse |
Permian (Asselian) |
Oxtrack Formation |
A member of Productida belonging to the superfamily Proboscidelloidea and the family Paucispinauriidae. |
|||
|
Sp. nov |
Valid |
Waterhouse |
Permian |
A member of Productida belonging to the superfamily Proboscidelloidea and the family Paucispinauriidae. |
||||
|
Sp. nov |
Valid |
Ma & Wang in Wang et al. |
Devonian (Famennian) |
Senzeille Formation |
A member of Spiriferida belonging to the family Cyrtospiriferidae. |
|||
|
Plicapustula (Paraplicapustula) magna[70] |
Sp. nov |
Valid |
Ma & Wang in Wang et al. |
Devonian (Famennian) |
Senzeille Formation |
A member of Spiriferida belonging to the family Cyrtospiriferidae. |
||
|
Ssp. nov |
Baranov & Blodgett |
Devonian (Pragian) |
Soda Creek Limestone |
Published online in 2024, but the issue date is listed as December 2023. |
||||
|
Sp. nov |
Baranov & Blodgett |
Devonian (Pragian) |
Soda Creek Limestone |
Published online in 2024, but the issue date is listed as December 2023. |
||||
|
Sp. nov |
Valid |
MacFarlan |
Late Triassic (Rhaetian) |
A member of Terebratulida belonging to the family Angustothyrididae. |
||||
|
Gen. et sp. nov |
Baranov & Blodgett |
Devonian (Pragian) |
Soda Creek Limestone |
Genus includes new species R. lata. Published online in 2024, but the issue date is listed as December 2023. |
||||
|
Sp. nov |
Jahangir et al. |
Ordovician |
Tungtzu Formation |
A member of Siphonotretida. |
||||
|
Sp. nov |
Baeza-Carratalá, Berrocal-Casero & García Joral |
Cretaceous (Albian–Cenomanian transition) |
Represa Formation |
A member of Terebratulida belonging to the family Sellithyrididae. |
||||
|
Sp. nov |
Valid |
Ma & Wang in Wang et al. |
Devonian (Famennian) |
Senzeille Formation |
A member of Spiriferida belonging to the family Cyrtospiriferidae. |
|||
|
Sp. nov |
Valid |
Feldman et al. |
Middle Jurassic (Callovian) |
A member of Terebratulida belonging to the family Postepithyrididae. |
||||
|
Sp. nov |
Valid |
MacFarlan |
Early Jurassic |
A member of Spiriferinida belonging to the family Spiriferinidae. |
||||
|
Spiriferina sophiaealbae[54] |
Sp. nov |
Valid |
MacFarlan |
Early Jurassic |
A member of Spiriferinida belonging to the family Spiriferinidae. |
|||
|
Sp. nov |
Wu et al. |
Early Triassic (Olenekian) |
Nanpanjiang Basin |
A member of Terebratulida belonging to the family Dielasmatidae. |
||||
|
Sp. nov |
Valid |
Waterhouse & Lee in Lee et al. |
Permian (Kungurian) |
Snapper Point Formation |
||||
|
Ssp. nov |
Valid |
Waterhouse |
Permian |
Mangarewa Formation |
A member of Productida belonging to the superfamily Proboscidelloidea and the family Paucispinauriidae. |
|||
|
Sp. nov |
Valid |
Bitner et al. |
Oligocene (Rupelian) |
Lower Red Formation |
A species of Thecidellina. |
|||
|
Sp. nov |
Valid |
MacFarlan |
Late Triassic (Rhaetian) |
A member of Terebratulida belonging to the family Dielasmatidae. |
||||
|
Tibetothyris johnstoni[66] |
Sp. nov |
Valid |
MacFarlan |
Late Triassic (Rhaetian) |
A member of Terebratulida belonging to the family Dielasmatidae. |
|||
|
Sp. nov |
Valid |
Halamski & Baliński |
Devonian (Givetian) |
Skały Formation |
A member of Spiriferida belonging to the family Reticulariidae. |
|||
|
Sp. nov |
Valid |
Wenndorf |
Devonian (Emsian) |
A member of Rhynchonellida belonging to the superfamily Rhynchotrematoidea and the family Sapphicorhynchidae. |
||||
|
Sp. nov |
Valid |
MacFarlan |
Late Triassic (Rhaetian) |
A member of Terebratulida belonging to the family Zeilleriidae. |
Brachiopod research
[edit]- Body and trace fossils indicative of early colonization of deep-marine turbidite settings by lingulid brachiopods are reported from the Ordovician Agüeira Formation (Spain) by Paz et al. (2023).[76]
- A study on the diversification dynamics of brachiopods and bivalves throughout their evolutionary histories is published by Guo et al. (2023), who interpret their findings as indicating that the switch from brachiopods to bivalves as major seabed organisms was unlikely to be caused by competitive exclusion of brachiopods by bivalves, but rather was likely caused by loss of brachiopod diversity in the Permian–Triassic extinction event and by bivalve diversification in the Cretaceous and Cenozoic that wasn't matched by brachiopods.[77]
- A study on the morphological diversity of lingulid brachiopods throughout the Phanerozoic is published by Liang et al. (2023), who find that Phanerozoic mass extinctions disproportionally wiped out lingulids that were not infaunal, and interpret the limited morphological and ecological diversity of modern lingulids as mainly resulting from differential effects of mass extinctions rather than from deterministic processes such as natural selection.[78]
Molluscs
[edit]Echinoderms
[edit]New echinoderm taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Valid |
Ausich & Wilson |
Carboniferous (Tournaisian) |
Cuyahoga Formation |
A camerate crinoid belonging to the group Monobathrida and the family Coelocrinidae. |
|||
|
Sp. nov |
Valid |
Courville et al. |
Late Pliocene—Early Pleistocene |
A species of Arbacia. |
||||
|
Sp. nov |
Valid |
Courville et al. |
Pliocene |
A species of Arbacia. |
||||
|
Sp. nov |
Valid |
Courville et al. |
Miocene |
A species of Arbacia. |
||||
|
Sp. nov |
Valid |
Courville et al. |
Pliocene |
A species of Arbacia. |
||||
|
Sp. nov |
Thuy, Piñuela & García-Ramos |
Early Jurassic (Sinemurian) |
Rodiles Formation |
A brittle star belonging to the order Ophiacanthida and the suborder Ophiodermatina. |
||||
|
Gen. et comb. nov |
Valid |
Zamora, Wright & Nohejlová |
Cambrian (Wuliuan) |
A member of the group Cincta belonging to the family Sucocystidae. The type species is "Asturicystis" havliceki Fatka & Kordule (2001). |
||||
|
Sp. nov |
Valid |
Ausich & Wilson |
Carboniferous (Tournaisian) |
Cuyahoga Formation |
A camerate crinoid belonging to the group Monobathrida and the family Actinocrinitidae. |
|||
|
Sp. nov |
Valid |
Ausich et al. |
Silurian (Homerian) |
A crinoid belonging to the group Monobathrida and the family Eucalyptocrinitidae. |
||||
|
Calliocrinus poepplemani[84] |
Sp. nov |
Valid |
Ausich et al. |
Silurian (Homerian) |
Laurel Formation |
A crinoid belonging to the group Monobathrida and the family Eucalyptocrinitidae. |
||
|
Sp. nov |
Schlüter et al. |
Late Cretaceous (Santonian) |
A sea urchin belonging to the family Phymosomatidae. |
|||||
|
Sp. nov |
Gale et al. |
Early Cretaceous (Albian) |
Enokuchi Formation |
An astropectinid starfish. |
||||
|
Sp. nov |
Valid |
Ausich & Wilson |
Carboniferous (Tournaisian) |
Cuyahoga Formation |
A camerate crinoid belonging to the group Monobathrida and the family Actinocrinitidae. |
|||
|
Sp. nov |
Valid |
Saucède et al. |
Early Triassic (Olenekian) |
A crinoid belonging to the group Articulata and the family Dadocrinidae. |
||||
|
Sp. nov |
Valid |
Ausich & Wilson |
Carboniferous (Tournaisian) |
Cuyahoga Formation |
A crinoid belonging to the group Cladida and the family Decadocrinidae. |
|||
|
Decadocrinus laevis[79] |
Sp. nov |
Valid |
Ausich & Wilson |
Carboniferous (Tournaisian) |
Cuyahoga Formation |
A crinoid belonging to the group Cladida and the family Decadocrinidae. |
||
|
Sp. nov |
Valid |
Zamora & Gutiérrez-Marco |
Silurian (Ludlow) |
Llagarinos Formation |
A member of Soluta belonging to the group Dendrocystitida and the family Dendrocystitidae. |
|||
|
Sp. nov |
Valid |
Gale |
Late Cretaceous (Cenomanian) |
Chalk Group |
A crinoid belonging to the family Roveacrinidae. |
|||
|
Sp. nov |
Valid |
Müller & Hahn |
Devonian (Emsian) |
A starfish belonging to the group Hadrosida and the family Xenasteridae. |
||||
|
Devonaster wenndorfi[90] |
Sp. nov |
Valid |
Müller & Hahn |
Devonian (Emsian) |
A starfish belonging to the group Hadrosida and the family Xenasteridae. |
|||
|
Gen. et sp. nov |
Valid |
Gale |
Late Cretaceous (Cenomanian) |
Chalk Group |
A crinoid belonging to the family Roveacrinidae. The type species is D. minutus. |
|||
|
Nom. nov |
Ceccolini & Cianferoni |
Ordovician |
A replacement name for Blastocystis Jaekel (1918). Sałamatin & Kaczmarek (2022) coined a replacement name Astroblastocystis for the same genus.[92] |
|||||
|
Nom. nov |
In press |
Ceccolini & Cianferoni |
Devonian |
A replacement name for Hymenosoma Lehmann (1957). |
||||
|
Gen. et sp. nov |
Valid |
Park & Lee |
Ordovician (Darriwilian) |
Jigunsan Formation |
A camerate crinoid belonging to the group Diplobathrida and the family Rhodocrinitidae. Genus includes new species G. pentagrammos. |
|||
|
Sp. nov |
Stiller |
Middle Triassic (Anisian) |
A holocrinid crinoid. |
|||||
|
Gen. et sp. nov |
Valid |
Reddy et al. |
Devonian (Pragian to Emsian) |
Baviaanskloof Formation |
A brittle star belonging to the group Oegophiurida and the family Encrinasteridae. The type species is K. spinosus. |
|||
|
Sp. nov |
Schlüter et al. |
Late Cretaceous (Santonian) |
A sea urchin belonging to the family Micrasteridae. |
|||||
|
Gen. et sp. nov |
Dupichaud et al. |
Ordovician (Tremadocian) |
A member of Soluta belonging to the group Syringocrinida and the family Minervaecystidae. The type species is N. agterbosi. |
|||||
|
Sp. nov |
Valid |
Kalyakin & Barsukov |
Early Cretaceous (Albian) |
A sea urchin belonging to the group Cassiduloida and the family Nucleolitidae. |
||||
|
Sp. nov |
Valid |
Thuy & Numberger-Thuy |
Late Cretaceous (Campanian) |
A brittle star, a species of Ophiocoma. |
||||
|
Sp. nov |
Gale et al. |
Early Cretaceous (Albian) |
Enokuchi Formation |
A hemieuryalid brittle star. |
||||
|
Nom. nov |
In press |
Ceccolini & Cianferoni |
Carboniferous |
A replacement name for Cycloscapus Moore & Jeffords (1968). |
||||
|
Sp. nov |
Valid |
Sweeney & Sumrall |
Ordovician (Sandbian) |
A rhombiferan belonging to the group Glyptocystitida and the family Pleurocystitidae. |
||||
|
Sp. nov |
Valid |
Gale |
Late Cretaceous (Cenomanian) |
Grey Chalk Subgroup of the Chalk Group |
A starfish, a species of Pteraster. Published online in 2022, but the issue date is listed as February 2023.[100] |
|||
|
Gen. et sp. nov |
Valid |
Müller & Ausich |
Devonian |
Seifen Formation |
A periechocrinid camerate crinoid. The type species is P. hardyi. |
|||
|
Sp. nov |
Valid |
Gale |
Late Cretaceous (Cenomanian) |
Aït Lamine Formation |
A crinoid belonging to the family Roveacrinidae. |
|||
|
Roveacrinus precarinatus[89] |
Sp. nov |
Valid |
Gale |
Late Cretaceous (Cenomanian) |
Chalk Group |
A crinoid belonging to the family Roveacrinidae. |
||
|
Gen. et sp. nov |
Poatskievick Pierezan, Gale & Fauth |
Early Cretaceous (Aptian–Albian) |
Sergipe-Alagoas Basin |
A crinoid belonging to the family Roveacrinidae. Genus includes new species S. reticulatus. |
||||
|
Sp. nov |
In press |
Ishida et al. |
Pliocene |
Hatsuzaki Formation |
A brittle star. |
|||
|
Sp. nov |
Valid |
Gale |
Late Cretaceous (Cenomanian) |
Chalk Group |
A crinoid belonging to the family Roveacrinidae. |
|||
|
Sp. nov |
Salamon et al. |
Early Jurassic (Pliensbachian) |
A cyrtocrinid. |
|||||
|
Gen. et sp. nov |
Ishida et al. |
Late Triassic (Carnian) |
A brittle star belonging to the group Ophioleucida. Genus includes new species T. meensis. Published online in 2022, but the issue date is listed as April 2023.[105] |
|||||
|
Gen. et sp. nov |
Valid |
Fau & Villier |
Late Cretaceous (Cenomanian) |
A stem zoroasterid. The type species is V. guerangeri. |
Echinoderm research
[edit]- Cole, Wright & Thompson (2023) experimentally confirm that ratios of seawater magnesium and calcium have a profound effect on short-term regeneration rates in extant brittle star Ophioderma cinereum, but find no evidence of a significant relationship between changes of seawater magnesium and calcium ratios and long-term changes of echinoderm biodiversity over the past 500 million years.[107]
- Evidence from a soft robotic representation and computer simulation, interpreted as indicating that pleurocystitids were likely able to move on the sea bottom by using their muscular stem that pushed the animal forward, is presented by Desatnik et al. (2023).[108]
- Álvarez-Armada et al. (2023) describe a specimen of Hyperoblastus reimanni preserved with structures interpreted as three larvae and a gonad, and interpret this finding as indicative of the presence of sexual dimorphism in blastoids, as well as of early evolution of internal brooding of larvae in this group.[109]
- A study on the evolution of plate systems in the calyx of crinoids, based on data from early crinoids from Tremadocian, is published by Guensburg, Mooi & Mongiardino Koch (2023).[110]
- A study on the ontogeny of Erisocrinus typus, based on data from fossil material representing a growth series from the Carboniferous Barnsdall Formation (Oklahoma, United States), is published by Hernandez Gomez et al. (2023).[111]
- Gorzelak et al. (2023) report the presence of microstructure similar to the diamond-type triply periodic minimal surfaces in the skeleton of a specimen of Haplocrinites from Devonian, similar to microstructure reported in extant Protoreaster nodosus, and representing the oldest record of such microstructure in echinoderms reported to date.[112]
- The oldest fossil material of members of the genus Percevalicrinus reported to date is described from the Lower Jurassic deposits in the western Saharan Atlas (Algeria) by Salamon et al. (2023).[113]
- Kolata et al. (2023) report the discovery of new specimens of Cyclocystoides scammaphoris from the Ordovician Platteville Formation (Illinois), Plattin and Decorah groups (Missouri) and Lebanon Limestone (Tennessee), providing new information on the anatomy of this cyclocystoid.[114]
- Evidence from deep-sea sediment samples interpreted as indicative of continuous record of deep-sea Atelostomata dating back to the Early Cretaceous is presented by Wiese et al. (2023).[115]
- The youngest stenuroid asterozoan specimen reported to date is described from the Permian (Wordian-Capitanian) Las Delicias Formation (Mexico) by Sour-Tovar, Quiroz-Barroso & Martín-Medrano (2023).[116]
- Thuy et al. (2023) report the discovery of an assemblage of brittle star microfossils from Carboniferous deep-water sediments of Oklahoma (United States), including fossils of basal representatives of Amphilepidida and Ophioscolecida, and interpret this finding as indicating that a significant part of the early diversification of the brittle star crown group might have taken place in deep-water settings.[117]
Hemichordates
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Valid |
Maletz |
Ordovician |
A graptolite belonging to the family Didymograptidae. |
||||
|
Baltograptus novus[118] |
Sp. nov |
Valid |
Maletz |
Ordovician |
A graptolite belonging to the family Didymograptidae. |
|||
|
Sp. nov |
Valid |
Maletz |
Ordovician |
A graptolite belonging to the family Didymograptidae. |
||||
|
Sp. nov |
In press |
Maletz |
Silurian |
A graptolite belonging to the family Retiolitidae. |
||||
|
Gothograptus osgaleae[119] |
Sp. nov |
In press |
Maletz |
Silurian |
A graptolite belonging to the family Retiolitidae. |
|||
|
Sp. nov |
Valid |
Maletz |
Ordovician |
A graptolite belonging to the family Kinnegraptidae/Sigmagraptidae. |
||||
|
Sp. nov |
In press |
Maletz |
Silurian |
A graptolite belonging to the family Retiolitidae. |
||||
|
Gen. et sp. nov |
Valid |
Briggs & Mongiardino Koch |
Silurian |
A pterobranch placed in its own new family Rotaciurcidae within Cephalodiscida. The type species is R. superbus. |
||||
|
Sp. nov |
Valid |
Maletz |
Ordovician |
A graptolite belonging to the family Phyllograptidae. |
Hemichordate research
[edit]- Nanglu et al. (2023) report the discovery of an orthocone cephalopod phragmocone from the Ordovician Fezouata Formation (Morocco) which was extensively populated by rhabdopleurid-like epibionts after the death of the cephalopod, interpreted as evidence of the use of mollusc shells as hard substrates by hemichordates dating back nearly 480 million years ago;[121] however, their identification of purported epibionts is subsequently contested by Maletz & Gutiérrez-Marco (2024), who argue that the studied specimens might be pseudo-colonial tubaria of cephalodiscid-like epibenthic pterobranchs instead.[122]
- Lopez et al. (2023) describe graptolite fossil material from the Silurian Rinconada Formation (Argentina), representing the first Pridolian graptolite assemblage from South America reported to date, and possibly providing evidence of faunal recovery interval after the Kozlowskii-Lau Event.[123]
Conodonts
[edit]New conodont taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Gen. et sp. nov |
Rueda & Albanesi |
Cambrian (Furongian) |
Lampazar Formation |
Genus includes A. anellus. |
||||
|
Sp. nov |
Lu |
Devonian |
Nahkaoling Formation |
A member of the family Spathognathodontidae. |
||||
|
Sp. nov |
Albanesi et al. |
Ordovician (Darriwilian) |
Santa Gertrudis Formation |
|||||
|
Sp. nov |
Albanesi et al. |
Ordovician (Darriwilian) |
Santa Gertrudis Formation |
|||||
|
Gen. et sp. nov |
Albanesi et al. |
Ordovician (Darriwilian) |
Santa Gertrudis Formation |
Genus includes new species G. elegantissimus. |
||||
|
Sp. nov |
Chen et al. |
|||||||
|
Sp. nov |
Valid |
Izokh |
Devonian (Givetian) |
|||||
|
Icriodus edentatus[129] |
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
|||
|
Icriodus kuzbassiensis[128] |
Sp. nov |
Valid |
Izokh |
Devonian (Givetian) |
||||
|
Icriodus lebedyankensis[128] |
Sp. nov |
Valid |
Izokh |
Devonian (Givetian) |
||||
|
Sp. nov |
Disputed |
Lyu & Zhao in Lyu et al. |
Early Triassic |
A member of the family Gondolellidae belonging to the subfamily Neogondolellinae. Argued to be a senior synonym of Neospathodus bevelledi by Wu et al. (2023);[131] on the other hand, Leu (2024) argued that N. yangtzeensis is a junior synonym of N. bevelledi.[132] |
||||
|
Sp. nov |
Lyu & Zhao in Lyu et al. |
Early Triassic |
||||||
|
Ssp. nov |
Valid |
Lu in Lu et al. |
Devonian (Lochkovian) |
Nahkaoling Formation |
||||
|
Sp. nov |
Valid |
Pei & Ba |
||||||
|
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
A member of Prioniodontida belonging to the family Icriodontidae. |
|||
|
Pelekysgnathus ziqiuensis[129] |
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
A member of Prioniodontida belonging to the family Icriodontidae. |
||
|
Gen. et comb. nov |
Valid |
Zhen |
Ordovician |
Genus erected to substitute Texania Pohler (1994), which is a junior homonym of Texania Casey (1909). Includes species previously assigned to the genus Texania, as well as species previously assigned to the genus Fahraeusodus other than F. adentatus. |
||||
|
Ssp. nov |
In press |
Suttner et al. |
Devonian |
Indert Formation |
||||
|
Polygnathus dispersus[129] |
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
|||
|
Polygnathus peltatus[129] |
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
|||
|
Polygnathus sagittiformis[129] |
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
|||
|
Polygnathus wuqingnaensis[137] |
Sp. nov |
Huang et al. |
Devonian (Famennian) |
Wuqingna Formation |
||||
|
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
A member of the family Polygnathidae. |
|||
|
Polylophodonta nodulosa[129] |
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
A member of the family Polygnathidae. |
||
|
Sp. nov |
In press |
Yuan & Sun |
Devonian (Famennian) |
Xiejingsi Formation |
A member of the family Polygnathidae. |
|||
|
Sp. nov |
Zhen et al. |
Ordovician |
Yinchufu Formation |
|||||
|
Gen. et 2 sp. nov |
Albanesi et al. |
Ordovician (Darriwilian) |
Santa Gertrudis Formation |
Genus includes new species P. cactus and P. spinatus. |
||||
|
Sp. nov |
Plotitsyn & Zhuravlev |
Carboniferous (Tournaisian) |
||||||
|
Sp. nov |
Albanesi et al. |
Ordovician (Darriwilian) |
Santa Gertrudis Formation |
|||||
|
Sp. nov |
Valid |
Lu in Lu et al. |
Devonian (Lochkovian) |
Nahkaoling Formation |
Conodont research
[edit]- A study on the size-frequency distribution of the P1 elements of members of the genera Palmatolepis, Ancyrodella and Polygnathus during the late Frasnian and the Famennian is published by Girard et al. (2023), who don't confirm the temperature-size rule as a general rule explaining size variation in the studied fossils.[140]
- Wu et al. (2023) report the discovery of an abundant conodont community in the Lower Triassic strata in the Zhangjiawan stratigraphic succession (Yuan'an County, Hubei, China), and interpret this finding as suggesting that the studied area might have been a refuge area for the Early Triassic conodont communities and marine ecosystem in general, as other Lower Triassic strata nearby yield only rare conodonts.[141]
- Evidence indicating that co-occurring Late Triassic conodonts Metapolygnathus communisti and Epigondolella rigoi differed in their diets is presented by Kelz et al. (2023).[142]
- A study on the diversity and biostratigraphy of late Norian conodont faunas from the Dashuitang and Nanshuba formations in the Baoshan area (Yunnan, China) is published by Zeng et al. (2023), who report evidence of a decline of conodont diversity during the late Norian, interpreted by the authors as the first crisis of the protracted suite of end-Triassic conodont extinctions.[143]
- Evidence from the Kastuyama section in the Inuyama area in Honshu (Japan), argued to be indicative of the survival of the conodont species Misikella posthernsteini into the Early Jurassic, is presented by Du et al. (2023).[144]
Fish
[edit]Amphibians
[edit]New amphibian taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Gen. et sp. nov |
Hart et al. |
Triassic |
A chigutisaurid temnospondyl. The type species is A. supinatus. |

| ||||
| Compsocerops tikiensis[146] | Sp. nov. | Chakravorti & Sengupta | Late Triassic | Tiki Formation | A member of Chigutisauridae. | |||
| Funcusvermis[147] | Gen. et sp. nov. | Valid | Kligman et al. | Late Triassic (Norian) | Chinle Formation | A stem-caecilian. The type species is F. gilmorei. | 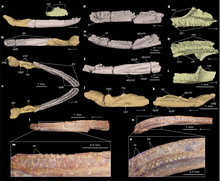
| |
|
Gen. et sp. nov |
Valid |
Zhang et al. |
Early Cretaceous |
A frog, possibly a basal member of Lalagobatrachia. The type species is G. qilianensis. |
||||
|
Gen. et sp. nov |
Valid |
Lemierre et al. |
Late Cretaceous (Coniacian–Santonian) |
A frog belonging to the family Pipidae. The type species is I. ragei. |
||||
|
Gen. et sp. nov |
Valid |
Skutschas et al. |
Early Cretaceous (Aptian) |
A member of the family Karauridae. The type species is K. sola. |
||||
|
Sp. nov |
Valid |
Turazzini & Gómez |
Late Miocene-Early Pliocene |
Tunuyán Formation |
A ceratophryid frog, a species of Lepidobatrachus. |

| ||
|
Gen. et sp. nov |
Santos, Carvalho & Zaher |
Late Cretaceous (Campanian) |
A neobatrachian frog, probably with affinities with hyloids. The type species is M. navai. |
|||||
|
Gen. et sp. nov |
Werneburg et al. |
Permian |
Boskovice Basin |
A branchiosaurid temnospondyl. The type species is P. kochovi. |
||||
|
Gen. et sp. nov |
Gee, Beightol & Sidor |
Triassic |
A lapillopsid temnospondyl. The type species is R. isbelli. |
|||||
|
Gen. et comb. nov |
Valid |
Schoch & Werneburg |
Permian (possibly Sakmarian) |
Niederhäslich–Schweinsdorf Formation |
A branchiosaurid temnospondyl. The type species is "Branchiosaurus" gracilis Credner (1881). |
Amphibian research
[edit]- New reconstruction of the skull of Crassigyrinus scoticus is presented by Porro, Rayfield & Clack (2023).[156]
- Pardo (2023) redescribes the anatomy of the neurocranium of Archeria crassidisca, based on data from a previously unreported partial braincase, and interprets embolomeres as more likely to be stem-tetrapods than stem-amniotes.[157]
- A study on the morphology and ossification sequences of carpus and tarsus in basal stereospondylomorphs, providing evidence of variability in the development of the mesopodium, is published by Witzmann & Fröbisch (2023).[158]
- Werneburg et al. (2023) describe twelve larvae of Sclerocephalus from the Permian (Asselian) Meisenheim Formation (Germany), providing new information on the earliest stages of the ontogeny of this temnospondyl.[159]
- Groenewald et al. (2023) describe body impressions and associated swim trails of rhinesuchid temnospondyls from the Permian Karoo Basin (South Africa), providing evidence that rhinesuchids used their tails for propulsion and held their legs tucked in next to the body while swimming.[160]
- A study comparing the probable maximum sizes that could be reached by specimens belonging to the Early Triassic temnospondyl taxa from Eastern Europe is published by Morkovin (2023), who reports the discovery of an unusually large lower jaw of Vladlenosaurus alexeyevi from the Skoba locality (Komi Republic, Russia), and argues that the size differences characteristic of the standard adult states of the studied temnospondyl taxa were likely reduced in individuals belonging to very late age categories.[161]
- New information on the anatomy of Mastodonsaurus cappelensis is presented by Schoch et al. (2023), who report the presence of anatomical differences between M. cappelensis and the stratigraphically younger M. giganteus, indicating that the latter species adapted to feed on a wider range of prey.[162]
- A study on the histology of large temnospondyl humeri from the Late Triassic Krasiejów site (Poland) is published by Teschner et al. (2023), who report that the humeri of Cyclotosaurus intermedius and Metoposaurus krasiejowensis might show only minor differences in morphology, making histology a valuable tool for taxonomic assignment.[163]
- A study on the skull morphology of Koskinonodon perfectum, Dutuitosaurus ouazzoui, Metoposaurus diagnosticus and Eocyclotosaurus appetolatus, providing evidence which might be indicative of the presence of sexual dimorphism in the studied taxa, is published by Rinehart & Lucas (2023).[164]
- Review of the fossil record of the genus Mioproteus in Southeastern Europe is published by Syromyatnikova (2023).[165]
- Skutschas et al. (2023) describe salamander dentaries from the Lower Cretaceous Teete locality (Batylykh Formation; Sakha, Russia) representing the northernmost record of non-karaurid salamanders in the Mesozoic reported to date.[166]
- Bourque, Stanley & Hulbert (2023) describe two partial vertebrae of a member of the genus Batrachosauroides from the Clarendonian Love Bone Bed (Florida, United States), representing the latest record of a member of this genus reported to date.[167]
- A study on the origin of the unique body plan of frogs, based on data from extant and fossil taxa, is published by Pérez-Ben, Lires & Gómez (2023), who interpret their findings as indicative of early diversification (resulting in diversity of locomotor modes in Jurassic stem frogs) followed by period of reduced morphological diversity and repeated convergent evolution of limb proportions and locomotor capabilities, and do not consider the interpretation of the body plan of frogs as resulting from an adaptation the ancestral frogs to jumping to be strongly supported by the fossil record.[168]
- Description of the anatomy of the metamorphosing larvae, juveniles and fully grown adults of Genibatrachus is published by Roček, Dong & Wang (2023).[169]
- The easternmost and the youngest frog remains from the Late Cretaceous of Asia reported to date are described from the Maastrichtian dinosaur locality in the city of Blagoveshchensk (Amur Oblast, Russia) by Skutschas et al. (2023).[170]
- Báez & Turazzini (2023) redescribe the holotype of Avitabatrachus uliana, reinterpreting the urostyle as not fully fused to the sacral vertebra, and identify additional fossil material preserved in the slab including the holotype, interpreted as likely remains of a metamorphosing tadpole of A. uliana.[171]
- Vallejo-Pareja et al. (2023) describe the first pre-Quaternary fossils referred to Eleutherodactylus from Florida, and interpret this finding as indicating that by the Late Oligocene Eleutherodactylus was already established in North America before colonizing Central America.[172]
- Georgalis, Prendini & Roček (2023) describe new fossil material of Thaumastosaurus from the Eocene Quercy Phosphorites Formation (France), and report evidence of diversity of morphotypes in the fossil material of Thaumastosaurus which might be indicative of the presence of cryptic taxa.[173]
- Lemierre et al. (2023) describe a skeleton of a member of the genus Pelophylax from the lowest Oligocene of Chartres-de-Bretagne (western France), representing one of the oldest occurrences of the genus reported to date.[174]
- Jansen et al. (2023) describe the first frog fossils from the Paleogene and early Neogene sites in Peruvian Amazonia, including fossil material of pipids, as well representatives of different lineages of Brachycephaloidea and probably also of the family Leptodactylidae.[175]
- Klembara et al. (2023) describe a specimen of Discosauriscus pulcherrimus from the Asselian Vrchlabí Formation, representing the oldest record of this species from the Czech Republic, and a specimen of Discosauriscus cf. pulcherrimus from the Upper Carboniferous Ilmenau Formation (Germany), representing the oldest record of the genus Discosauriscus and possibly the oldest seymouriamorph reported to date.[176]
- Bazzana-Adams et al. (2023) reconstruct the first virtual cranial endocast of Seymouria.[177]
- Barták & Ivanov (2023) describe an exceptionally well-preserved specimen of Sauropleura scalaris (including near-complete skull) from the upper Carboniferous deposits of Nýřany (Czech Republic), providing new information on the anatomy and ontogeny of this taxon.[178]
- Bulanov (2023) reinterprets putative bolosaurid "Bolosaurus" traati as a diadectomorph, transfers it to the genus Stephanospondylus, and considers Ambedus to be a non-diadectomorph tetrapod of uncertain affinities.[179]
- Calábková et al. (2023) describe tracks assigned to the ichnogenus Ichniotherium from the Permian (Asselian) Boskovice Basin (Czech Republic), including tracks with morphologies similar to footprints produced by Diadectes-like diadectomorphs, but with distances between the successive imprints similar to those seen in earlier-diverging diadectomorphs.[180]
Reptiles
[edit]Synapsids
[edit]Non-mammalian synapsids
[edit]New synapsid taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Gen. et sp. nov |
Valid |
Mueller et al. |
Late Triassic |
A placeriine kannemeyeriiform dicynodont. The type species is A. boreni. |
||||
|
Gen. et sp. nov |
Valid |
Sidor |
A burnetiid burnetiamorph. The type species is B. bulborhynchus. |
|||||
|
Gen. et sp. nov |
Valid |
Kammerer |
Permian (Guadalupian) |
A scylacosaurid therocephalian. The type species is E. vandenheeveri. |
||||
| Inostrancevia africana[184] | Sp. nov | Valid | Kammerer et al. | Permian | Balfour Formation | A gorgonopsid. | ||
|
Sp. nov |
Valid |
Shi & Liu |
Permian |
A dicynodont. |
||||
|
Gen. et sp. nov |
Valid |
Suchkova, Golubev & Shumov |
Permian |
A therocephalian. The type species is K. grechovi. Published online in 2023, but the issue date is listed as December 2022.[186] |
||||
|
Gen. et sp. nov |
Valid |
Mann et al. |
Carboniferous (Moscovian) |
Allegheny Group |
A member of the family Edaphosauridae. The type species is M. hovaneci. |
|||
|
Sp. nov |
Valid |
Jun & Abdala |
Permian (Lopingian) |
A whaitsiid therocephalian. |
||||
|
Gen. et sp. nov |
Valid |
Day & Kammerer |
Permian (Guadalupian) |
A proburnetiine burnetiamorph. The type species is N. brucei. |
||||
|
Gen. et sp. nov |
Valid |
Sidor |
Permian (Lopingian) |
A burnetiid burnetiamorph. The type species is P. litumbaensis. |
||||
| Santagnathus[190] | Gen. et sp. nov | Schmitt et al. | Late Triassic (Carnian) | Santa Maria Formation (Hyperodapedon Assemblage Zone) | A cynodont in the family Traversodontidae. The type species is S. mariensis | |||
| Woznikella[191] | Gen. et sp. nov | Valid | Szczygielski & Sulej | Late Triassic (Carnian–?Norian) |
Grabowa Formation | A dicynodont closely related to the family Stahleckeriidae. The type species is W. triradiata. |
Synapsid research
[edit]- A study on the evolution of the dentary size in non-mammalian synapsids is published by Harano & Asahara (2023), who find evidence indicative of an evolutionary trend for enlargement of dentary relative to the overall lower jaw size across all non-mammalian synapsids, regardless of their relation to mammals, but find no evidence for an evolutionary trend in dentary enlargement at the expense of the postdentary bones.[192]
- Studies on the evolution of the forelimb and hindlimb musculature of synapsids are published by Bishop & Pierce (2023).[193][194]
- A study on the ecomorphology of synapsids throughout their evolutionary history is published by Hellert et al. (2023), who find carnivory to be the ancestral dietary regime of major synapsid radiations, but also find that small body size was established as the common ancestral state of radiations as late as in the Late Triassic, near the origin of Mammaliaformes, and report the presence of derived traits in the ancestors of major synapsid radiations.[195]
- Calábková et al. (2023) describe tracks assignable to the ichnogenus Dimetropus and produced by "pelycosaur"-grade synapsids from the Permian (Asselian) Padochov and Letovice formations (Boskovice Basin, Czech Republic), including a specimen with preserved skin impressions, and providing new information on the diversity of the earliest Permian equatorial tetrapod faunas.[196]
- Maho, Bevitt & Reisz (2023) describe fossil material of Varanops brevirostris from the Dolese Brother Limestone Quarry (Oklahoma, United States), confirming the presence of this taxon at Richards Spur, and interpret this finding as indicating that, although less abundant than Cacops and Acheloma, V. brevirostris was not as rare taxon as previously thought.[197]
- Gônet et al. (2023) present a model which can be used to determine posture from humeral parameters in extant mammals, and use it to infer a sprawling posture for Dimetrodon natalis.[198]
- Bazzana-Adams, Evans & Reisz (2023) describe the brain and inner ear of Dimetrodon loomisi, and interpret their findings as indicating that Dimetrodon was sensitive to a greater range of frequencies beyond the ultra-low-frequency ground-borne sounds anticipated in previous estimates.[199]
- Partial humerus of a synapsid of uncertain affinities, with anatomical traits blurring the distinction between the "pelycosaur"-grade synapsids and therapsids, is described from the Permian (Capitanian) Main Karoo Basin (South Africa) by Bishop et al. (2023).[200]
- An almost complete skull of Pampaphoneus biccai, providing new information on the anatomy of this species, is described from the Permian Rio do Rasto Formation (Brazil) by Santos et al. (2023).[201]
- Benoit, Norton & Jirah (2023) describe the maxillary canal of Jonkeria truculenta, reporting that is structure shares more similarities with the maxillary canal of the tapinocephalid Moschognathus than with that of Anteosaurus.[202]
- Rubidge, Day & Benoit (2023) report the first discovery of the fossil material of Colobodectes cluveri from the Grootfontein Member of the Abrahamskraal Formation (South Africa), providing correlation between strata of the Abrahamskraal Formation from the northwestern, western and southwestern part of the Karoo Basin.[203]
- Laaß & Kaestner (2023) report the presence of a system of turbinal ridges for attachment of respiratory and olfactory turbinates (strongly resembling the mammalian condition) in the skull of Kawingasaurus fossilis, and interpret this finding as supporting a convergent origin of endothermy in dicynodonts, possibly influenced by their fossorial habitat.[204]
- New information on the anatomy of Cistecephalus microrhinus is presented by Macungo et al. (2023), who interpret cistecephalids as probable ectotherms, and argue that purported turbinals such as those reported in Kawingasaurus fossilis by Laaß & Kaestner (2023)[204] are probably lines left by sediment infilling of the skull cavity.[205]
- Sidor, Mann & Angielczyk (2023) report the discovery of the fossil material of the gorgonopsian Gorgonops sp. and the dicynodont Endothiodon sp. from the Permian Madumabisa Mudstone Formation, indicating that the stratigraphic range of vertebrate-bearing horizons in southern Zambia includes not only Guadalupian Tapinocephalus Assemblage Zone-equivalent strata, but also Lopingian Endothiodon Assemblage Zone-age strata.[206]
- Bendel et al. (2023) describe a nearly complete skeleton of Gorgonops torvus from the Permian Endothiodon Assemblage Zone of the Karoo Basin (South Africa), and interpret the anatomy of the studied specimen as indicating that G. torvus was likely ambush predator, able to chase its prey over short distances.[207]
- The first Permian tetrapod fossils from the Omingonde Formation, Namibia are described and identified as a dicynodont and a gorgonopsian therapsids by Mocke et al. (2023), comparable to Tropidostoma and Lycaenops, respectively.[208]
- The holotypes of Dicynodon ingens and Scymnosaurus warreni are re-located and redescribed by Groenewald & Kammerer (2023), who re-identify them as specimens of Daptocephalus leoniceps and Moschorhinus kitchingi, respectively.[209]
- The craniomandibular anatomy of the therocephalian Olivierosuchus is re-described by Gigliotti et al. (2023), including for the first time the braincase and inner ear, revealing similar neuroanatomy to other non-mammalian therapsids.[210]
- A historical and taxonomic review of the Karoo and global record of non-mammalian cynodonts is published by Abdala et al. (2023).[211]
- Redescription of the holotype of Nythosaurus larvatus is published by Pusch et al. (2023), who interpret N. larvatus as a taxon distinct from Thrinaxodon liorhinus.[212]
- Kulik (2023) compares femoral histology of two specimens of Scalenodon angustifrons of the same size, report evidence of skeletal maturity in one of the studied specimens, and interprets her findings as indicative of a flexible growth strategy in S. angustifrons.[213]
- A study on the dentition of Charruodon tetracuspidatus is published by Hoffmann, Ribeiro & de Andrade (2023), who interpret the holotype specimen as representing an early ontogenetic stage, and consider C. tetracuspidatus to be a nomen dubium.[214]
- Hoffmann, de Andrade & Martinelli (2023) redescribe the skeletal anatomy of "Probelesodon" kitchingi, and transfer this species to the genus Chiniquodon.[215]
- Description of new lower jaw remains of Agudotherium gassenae from the Late Triassic of Brazil, providing new information on the anatomy of this taxon, and a study on its phylogenetic affinities is published by Kerber, Pretto & Müller (2023), who recover A. gassenae as the sister taxon of Prozostrodontia.[216]
- Stefanello et al. (2023) describe a new, complete and exceptionally well-preserved skull of Prozostrodon brasiliensis from the Upper Triassic strata in Brazil, and name a new endemic clade of South American cynodonts – Prozostrodontidae, including Prozostrodon and Pseudotherium.[217]
- A study on the endocranial anatomy of Prozostrodon brasiliensis and Therioherpeton cargnini is published by Kerber et al. (2023).[218]
- Lund, Norton & Benoit (2023) describe the postcranial, basicranial and palatal morphology of Diarthrognathus broomi.[219]
- A study on the evolution of cynodont skulls is published by Lautenschlager et al. (2023), who find no evindence for an increase in cranial strength and biomechanical performance during the cynodont–mammalian transition.[220]
- A study on tooth replacement pattern and deciduous teeth in Haldanodon exspectatus is published by Martin & Schultz (2023), who interpret the fossil material of Peraiocynodon inexpectatus, P. major and Cyrtlatherium canei as likely to be docodont milk teeth.[221]
- Averianov, Lopatin & Leshchinskiy (2023) reinterpret one of the supposed lower premolars of Sibirotherium rossicum as the first molariform, and consider S. rossicum to have five rather than six lower premolars.[222]
Mammals
[edit]Other animals
[edit]Other new animal taxa
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Valid |
Sánchez-Beristain, Rodrigo & Schlagintweit |
Early Cretaceous (Aptian-Albian) |
Tuburan Limestone |
A chaetetid demosponge. |
|||
|
Sp. nov |
Valid |
Luzhnaya et al. |
Cambrian |
A sponge of uncertain affinities. |
||||
|
Gen. et comb. nov |
Valid |
Grimes et al. |
Ediacaran |
Ediacara Member of the Rawnsley Quartzite |
An arboreomorph. The type species is "Rangea" longa Glaessner & Wade (1966). |
|||
|
Sp. nov |
Valid |
Peng et al. |
Cambrian (Wuliuan) |
|||||
|
Gen. et sp. nov |
Valid |
Chen et al. |
Chiungchussu Formation |
A sponge, possibly a member of Silicea. The type species is C. chunchengia. |
||||
|
Sp. nov |
Valid |
Peng et al. |
Cambrian (Wuliuan) |
Kaili Formation |
A chancelloriid. |
|||
|
Gen. et 9 sp. nov |
Valid |
Poinar in Luo et al. |
Cretaceous |
Burmese amber |
A collective genus erected for fossil nematodes belonging to the family Mermithidae. The name was used in earlier publications, but the taxon was formally named in 2023.[228] Genus includes new species C. incredibilis Luo & Poinar, C. calypta Luo & Poinar, C. adelphe Luo & Poinar, C. directa Luo & Poinar, C. longa Luo & Poinar, C. perforissi Luo & Poinar, C. manicapsoci Luo & Poinar, C psoci Luo & Poinar and C. cecidomyiae Luo & Poinar, as well as "Heleidomermis" libani Poinar et al. (1994), C. chironomae Poinar (2011), C. aphidophilus Poinar (2017) and C. protus Poinar & Buckley (2006). |
|||
|
Sp. nov |
Botting, Muir & Doyle |
Carboniferous (Pennsylvanian) |
A reticulosan sponge. |
|||||
| Didemnum cassianum[230] | Comb. nov | Valid | Paulsen & Thibault | Early Jurassic (Hettangian-Sinemurian) | "Llanbdr (Mochras Farm)" core | An Ascidiacea Tunicate. The type species is "Tetralithus cassianus" by Jafar (1983) | ||
|
Gen. et sp. nov |
Valid |
Wang et al. |
Yu'anshan Formation |
A member of Priapulida. The type species is E. sparios. |
||||
|
Gen. et sp. nov |
Valid |
Goñi et al. |
Erkhelnuur Formation |
A palaeoscolecid. The type species is F. egiinensis. |
||||
|
Gen. et sp. nov |
Valid |
Zhao, Li & Selden |
Wulongqing Formation |
A polychaete. The type species is G. bifurcus. |

| |||
|
Gen. et sp. nov |
Valid |
Davydov et al. |
Carboniferous (Gzhelian) |
Kosherovo Formation |
A calcareous sponge. The type species is G. cornigera. Published online in 2024, but the issue date is listed as December 2023. |
|||
|
Gen. et sp. nov |
Zhang & Smith in Zhang, Smith & Ren |
Cambrian Stage 3 |
Yu'anshan Formation |
Probably an annelid belonging to the group Sedentaria, related to the families Flabelligeridae and Acrocirridae. The type species is I. chengjiangensis. The name was used in earlier publications, but the taxon wasn't formally described before 2023.[235] |

| |||
|
Sp. nov |
Valid |
Świerczewska-Gładysz & Jurkowska |
Late Cretaceous (Campanian) |
A demosponge belonging to the family Phymatellidae. |
||||
|
Gen. et sp. nov |
Liu & Huang in Liu et al. |
Ordovician |
Madaoyu Formation |
A palaeoscolecid. Genus includes new species L. hunanensis. |
||||
|
Gen. et sp. nov |
Valid |
Botting & Muir |
Ordovician (Darriwilian) |
Gilwern Volcanic Formation |
A spoon worm belonging to the family Thalassematidae. The type species is L. suzannae. |
|||
|
Gen. et sp. nov |
Valid |
Pronzato & Manconi in Samant et al. |
Late Cretaceous–Paleocene |
Naskal intertrappean beds |
A demosponge belonging to the family Palaeospongillidae. The type species is L. antiqua Manconi & Samant. |
|||
|
Gen. et sp. nov |
Valid |
Nanglu et al. |
Cambrian (Drumian) |
A tunicate. The type species is M. thylakos. |
||||
|
Gen. et sp. nov |
Valid |
McCall |
Cambrian |
A lobopodian belonging to the family Kerygmachelidae. The type species is M. adustus. |

| |||
|
Gen. et sp. nov |
Valid |
Demidenko |
Cambrian |
Bayangol Formation |
Sclerites of an animal of uncertain affinities, belonging to the family Siphogonuchitidae. The type species is M. dentatus. |
|||
|
Sp. nov |
Valid |
Kočí, Milàn & Jäger |
Paleocene (Selandian) |
Kerteminde Marl Formation |
An annelid belonging to the family Serpulidae. |
|||
| Palaeoquadrum[230] | Gen. et sp. nov | Valid | Paulsen & Thibault | Early Jurassic (Sinemurian) | "Llanbdr (Mochras Farm)" core | An Ascidiacea Tunicate. the type species is P. ullmanni | ||
|
Gen. et sp. nov |
Wu & Reitner in Wu et al. |
Early Triassic |
A keratose sponge. The type species is P. kejiaoensis. |
|||||
|
Gen. et sp. nov |
Valid |
Jeon & Kershaw in Jeon et al. |
Ordovician (Katian) |
Beiguoshan Formation |
A clathrodictyid stromatoporoid. The type species is P. exililamellatum. |
|||
|
Gen. et sp. nov |
Valid |
Wierzbowski & Błażejowski |
Devionian (Famennian) |
A member of Chaetognatha of uncertain affinities. The type species is P. polonicus. |
||||
|
Gen. et comb. nov |
Valid |
Pisera, Bitner & Fromont |
Eocene |
Pallinup Formation |
A demosponge belonging to the family Phymaraphiniidae. The type species is "Discodermia" tabelliformis Chapman & Crespin (1934). |
|||
|
Sp. nov |
Valid |
Jeon in Jeon et al. |
Ordovician (Katian) |
Beiguoshan Formation |
A clathrodictyid stromatoporoid. |
|||
|
Gen. et sp. nov |
Li & Reitner |
Ordovician |
Kaochiapien Formation |
A demosponge belonging to the family Bubaridae. The type species is P. hemisphaeroidalis. |
||||
| Quadrifolium[230] | Gen. et sp. nov | Valid | Paulsen & Thibault | Early Jurassic (Sinemurian) | "Llanbdr (Mochras Farm)" core | An Ascidiacea Tunicate. The type species is Q. hesselboi | ||
|
Sp. nov |
Valid |
McNamara |
Late Cretaceous (Santonian to Campanian) |
Toolonga Calcilutite |
A polychaete belonging to the family Serpulidae. |
|||
|
Sp. nov |
Valid |
McNamara |
Late Cretaceous (Santonian to Campanian) |
Gingin Chalk |
A polychaete belonging to the family Serpulidae. |
|||
|
Sp. nov |
Valid |
McNamara |
Late Cretaceous (Maastrichtian) |
A polychaete belonging to the family Serpulidae. |
||||
|
Gen. et sp. nov |
Kimmig et al. |
Cambrian (Wuliuan) |
A polychaete. The type species is S. shurikeni. |
|||||
|
Sp. nov |
Valid |
Jeon & Kershaw in Jeon et al. |
Ordovician (Katian) |
Beiguoshan Formation |
A stromatoporellid stromatoporoid. |
|||
|
Sp. nov |
Vinn et al. |
Silurian |
A member of Tentaculita. |
|||||
|
Sp. nov |
Valid |
Botting, Muir & Ma |
Ordovician |
Gilwern Volcanic Formation |
A hexactinellid sponge belonging to the family Teganiidae. |
|||
|
Gen. et comb. nov |
Valid |
Pisera, Bitner & Fromont |
Eocene |
Pallinup Formation |
A demosponge belonging to the family Phymaraphiniidae. The type species is "Thamnospongia" subglabra Chapman & Crespin (1934). |
|||
|
Gen. et sp. nov |
Valid |
Cambrian (Wuliuan) |
A polychaete. The type species is U. comosa. |

| ||||
|
Sp. nov |
Muir & Gutiérrez-Marco |
Ordovician (Tremadocian) |
An animal of uncertain affinity, might be a benthic graptolite or a hydrozoan.[122] |
Other animal research
[edit]- A study on the paleobiology of Eoandromeda octobrachiata is published by Botha et al. (2023), who interpret E. octobrachiata as a benthic, sessile, radially symmetrical organism, and consider it unlikely that E. octobrachiata was a stem-ctenophore.[255]
- A study aiming to test the hypothesized feeding modes of Pectinifrons abyssalis is published by Darroch et al. (2023), who interpret their findings as supporting neither a suspension feeding or osmotrophic feeding habit, and indicating that rangeomorph fronds were organs adapted for oxygen uptake and gas exchange, rather than feeding.[256]
- New information on the paleobiology of Culmofrons plumosa, based on data from specimens from the Trepassey Formation (Canada), is presented by Pasinetti & McIlroy (2023), who interpret rangeomorph impressions observed in Culmofrons as likely bundles of branches in the process of separating from the organism, potentially indicating that rangeomorphs had true modularity and the ability to separate modules as an asexual reproductive strategy.[257]
- Purported fossil material of Dickinsonia reported from the Bhimbetka rock shelters in rocks of the Maihar Sandstone (India)[258] is reinterpreted as an impression resulting from decay of a modern beehive by Meert et al. (2023).[259]
- A study aiming to assess the validity of species distinctions in the genus Dickinsonia is published by Evans et al. (2023), who interpret their findings as indicative of the presence of two distinct species from South Australia, D. costata and D. tenuis.[260]
- New information on the body plan of Dickinsonia, based on data from the fossil material from the southeastern White Sea area (Russia), is presented by Ivantsov & Zakrevskaya (2023), who interpret the anatomy of Dickinsonia as indicative of its affinity to the urbilaterian.[261]
- Evidence indicative of the impact of oxygen expansion driven by sea-level oscillations on the speciation of early Cambrian reef-building archaeocyaths from the Siberian Craton is presented by Zhuravlev, Wood & Bowyer (2023).[262]
- Evidence from computational fluid dynamics simulations of digital models of Archaeolynthus porosus and Favilynthus mellifer, interpreted as indicating that the studied archaeocyaths could not have functioned effectively as predominantly passive suspension feeders but rather had to use active suspension feeding methods, is presented by Gibson et al. (2023).[263]
- Yun et al. (2023) describe new fossil material of Hyalosinica archaica from the Cambrian Niutitang Formation (China), indicating that H. archaica developed a long stalk used to lift the main body above the sediment surface and reach more oxic water, and interpret H. archaica as a member of the stem group of Hexactinellida.[264]
- Łukowiak et al. (2023) report the discovery of a diverse assemblage of Miocene sponge spicules from the Guadalquivir Basin (Spain), including sponges with affinities to extant taxa from the Indo-Pacific and Japanese waters, and interpret changes of distribution of the studied sponge taxa as likely resulting from the isolation of the Mediterranean and the Messinian salinity crisis.[265]
- Fossil material of Nenoxites from the Ediacaran Khatyspyt Formation (Russia), originally interpreted as trace fossils providing evidence of early bioturbation,[266] is argued to more likely represent body fossil coquina of Shaanxilithes-like tubular organisms by Psarras et al. (2023), who interpret Shaanxilithes-type body fossils as possible total group eumetazoans.[267]
- Yun, Reitner & Zhang (2023) describe new, well-preserved fossil material of the chancelloriid Dimidia simplex from the Cambrian Yu'anshan Formation (China), and consider Dimidia to be a taxon distinct from Allonnia.[268]
- Yang et al. (2023) reinterpret putative Cambrian bryozoan Protomelission as an early dasycladalean green alga, and conclude that there are no unequivocal bryozoans of Cambrian age;[269] however, in a subsequent study Xiang et al. (2023) present new information on the morphology of Protomelission, and consider it to be a scleritome of Cambroclavus, which in turn is considered by the authors to be a probable epitheliozoan-grade eumetazoan like the contemporaneous chancelloriids, unrelated to bryozoans or to dasycladalean algae.[270]
- A study on the body architecture of Xianguangia sinica is published by Zhao, Hou & Cong (2023), who interpret the putative "column" part of its body as formed by 18 tentacle-sheath complexes, and interpret X. sinica as a possible stem-ctenophore related to Dinomischus and Daihua.[271]
- A specimen of the Ordovian hyolith Elegantilites custos with an operculum showing regeneration after non-lethal predatory attack is described by Fatka, Valent & Budil (2023).[272]
- Parry et al. (2023) describe fossil material of Plumulites tafennaensis from the Ordovician (Katian) Upper Tiouririne Formation (Morocco), including aberrant shell plates interpreted as resulting from healed injuries, and consider the soft tissue that secreted the shell plate to be similar in morphology and size relative to the body to that seen in scaleworm elytra.[273]
- Putative anostracan crustacean Gilsonicaris rhenanus is reinterpreted as a polychaete by Gueriau, Parry & Rabet (2023).[274]
- New cycloneuralian microfossils, preserving a musculature system interpreted as indicative of a phylogenetic relationships with scalidophorans and possibly priapulans, are described from the Cambrian Kuanchuanpu Formation (China) by Zhang et al. (2023).[275]
- Putative early leech from the Silurian Brandon Bridge Formation (Waukesha Biota; Wisconsin, United States) is considered to be a member of Cycloneuralia of uncertain affinities by Braddy, Gass & Tessler (2023).[276]
- Wu, Pisani & Donoghue (2023) study the interrelationship between main groups of Panarthropoda, attempting to determine whether morphological datasets from the studies of extant and fossil panarthropod relationships published by Legg, Sutton & Edgecombe (2013),[277] Yang et al. (2016)[278] and Aria, Zhao & Zhu (2021)[279] can discriminate statistically between competing Tactopoda, Lobopodia and Protarthopoda hypotheses, and question the accuracy of morphology-based phylogenies of Panarthropoda that include fossil species.[280]
- Kihm et al. (2023) compare the morphology of tardigrades and Cambrian lobopodians, and argue that ancestral tardigrades likely had a Cambrian lobopodian–like morphology and shared most recent ancestry with the luolishaniids.[281]
- New fossil material of Rotadiscus grandis is reported from the Cambrian Chengjiang biota from Yunnan (China) by Li et al. (2023), who recover Rotadiscus as a stem-ambulacrarian, and argue that such deuterostome traits as post-anal region, gill bars and a U-shaped gut evolved through convergence rather than shared ancestry.[282]
- Yang et al. (2023) report the discovery a previously unrecognized structure between the third and fourth segments of the posterior section of the body of weakly sclerotized vetulicolians, and interpret this structure as an internal organ, possibly used for reproduction, excretion or digestion.[283]
- Yang et al. (2023) report the discovery of the fossil material of Herpetogaster collinsi from the Cambrian Balang Formation (China), representing the first record of this species from Gondwana, and interpret the distribution of H. collinsi in both Laurentia and Gondwana, coupled with its phylogenetic placement at the base of the ambulacrarian tree, as suggesting that the last common ancestor of the ambulacrarians might have already had a planktonic larval stage (or that such larvae developed multiple times within the Ambulacraria), which would have permitted dispersal over long distances.[284]
- Claims of the presence of cellular cartilages, fibrillin and subchordal rod in yunnanozoan fossils made by Tian et al. (2022)[285] are contested by He et al. (2023)[286] and Zhang & Pratt (2023).[287][288]
- Redescription of the holotype of Chamasaurus dolichognathus is published by Jenkins, Meyer & Bhullar (2023).[289]
- A study on the anatomy and affinities of Tullimonstrum gregarium is published by Mikami et al. (2023), who interpret T. gregarium as more likely to be a non-vertebrate chordate or a protostome than a vertebrate.[290]
Other organisms
[edit]Other new organisms
[edit]| Name | Novelty | Status | Authors | Age | Type locality | Location | Notes | Images |
|---|---|---|---|---|---|---|---|---|
|
Sp. nov |
Ghavidel-Syooki & Piri-Kangarshahi |
Ordovician |
Lashkarak Formation |
An acritarch. |
||||
|
Gen. et sp. nov |
Riedman et al. |
Late Paleoproterozoic |
Blue Hole Formation |
A eukaryotic microfossil. The type species is B. brigandinia. |
||||
|
Gen. et sp. nov |
Valid |
Peel |
Cambrian (Wulian) |
An organism of uncertain affinities, with similarities to wiwaxiid and annelid sclerites, thelodont scales and the foraminiferan Lagena. The type is species C. lagenamorpha. |
||||
|
Gen. et sp. nov |
Wang et al. |
Devonian (?Pragian-Emsian) |
Cangwu Formation |
A member of Arcellinida of uncertain affinities. The type species is C. ampulliformis. |
||||
|
Gen. et sp. nov |
Liu et al. |
Cambrian |
An acritarch, possibly a representative of an extinct group of early multicellular eukaryotes. The type species C. ornata. |
|||||
|
Sp. nov |
Ghavidel-Syooki & Piri-Kangarshahi |
Ordovician |
Lashkarak Formation |
A chitinozoan. |
||||
|
Gen. et sp. nov |
Xian, Eriksson & Zhang |
Cambrian |
An organism of uncertain affinities, possibly an alga. The type species is D. plana. |
|||||
|
Gen. et sp. nov |
Riedman et al. |
Late Paleoproterozoic |
Blue Hole Formation |
A eukaryotic microfossil. The type species is F. torsivum. |
||||
|
Gen. et sp. nov |
Chen et al. |
Early Mesoproterozoic |
Gaoyuzhuang Formation |
A multicellular eukaryote of uncertain affinities. The type species is G. qianxiensis. |
||||
|
Sp. nov |
Valid |
Vorob'eva & Petrov |
Ediacaran and early Cambrian |
Ura Formation |
An acritarch. |
|||
|
Gen. et comb. nov |
Riedman et al. |
Late Paleoproterozoic and Mesoproterozoic |
Dzhelindukon Formation |
A eukaryotic microfossil. The type species is "Valeria" elongata Nagovitsin (2009). |
||||
|
Sp. nov |
Peng et al. |
Early Jurassic (Pliensbachian) |
A palynomorph. Argued to be a possible ephippium of a cladoceran by Peng et al. (2023),[299] but this interpretation was rejected by Van Damme (2023).[300] |
|||||
|
Gen. et sp. nov |
Riedman et al. |
Late Paleoproterozoic |
Blue Hole Formation |
A eukaryotic microfossil. The type species is L. operculata. |
||||
|
Sp. nov |
Ghavidel-Syooki & Piri-Kangarshahi |
Ordovician |
Lashkarak Formation |
An acritarch. |
||||
|
Sp. nov |
Ghavidel-Syooki & Piri-Kangarshahi |
Ordovician |
Lashkarak Formation |
An acritarch. |
||||
|
Gen. et sp. nov |
Valid |
Kaczmarska & Ehrman |
Oligocene (Rupelian) |
Menilite Formation |
A member of Parmales. The type species is P. janusii. |
|||
|
Gen. et sp. nov |
Valid |
Kaczmarska & Ehrman |
Oligocene (Rupelian) |
Menilite Formation |
A parmalean-like eukaryote of uncertain affinities. The type species is P. radiata. |
|||
|
Gen. et sp. nov |
Disputed |
Skompski et al. |
Silurian |
A graptolite-like form of uncertain affinities. The type species is P. algaeoides. Argued by LoDuca (2024) to actually represent the uppermost siphons of mature thalli of the alga Voronocladus dryganti.[303] |
||||
|
Gen. et sp. nov |
Xian, Eriksson & Zhang |
Cambrian |
An organism of uncertain affinities, possibly an alga. The type species is Q. conica. |
|||||
|
Gen. et sp. nov |
Xian, Eriksson & Zhang |
Cambrian |
An organism of uncertain affinities, possibly an alga. The type species is S. spinosa. |
|||||
|
Gen. et sp. nov |
Riedman et al. |
Late Paleoproterozoic and Mesoproterozoic |
A eukaryotic microfossil. The type species is S. bombycinum. |
|||||
|
Sp. nov |
Chen et al. |
Early Mesoproterozoic |
Gaoyuzhuang Formation |
A multicellular eukaryote of uncertain affinities. |
||||
|
Tuanshanzia parva[297] |
Sp. nov |
Chen et al. |
Early Mesoproterozoic |
Gaoyuzhuang Formation |
A multicellular eukaryote of uncertain affinities. |
|||
|
Gen. et sp. nov |
Valid |
Martyshyn |
Ediacaran |
Studenitsa Formation |
An organism of uncertain affinities, possibly a benthic plant with similarities to green algae or a fossil of the polyp stage of a medusozoan. The type species is T. primitiva. |
|||
|
Gen. et sp. nov |
Valid |
Tang et al. |
Xiamaling Formation |
An organism with similarities to cyanobacteria. The type species is X. sideria. |
Other organism research
[edit]- A study on the taphonomy of fossils of 2.1-billion-years-old soft-bodied organisms from the Franceville basin (Gabon) is published by Ngwal'ghoubou Ikouanga et al. (2023).[306]

- Franz et al. (2023) describe the morphology and the internal structure of at least 1.5-billion-years-old organisms from the Volyn pegmatite field associated with the Korosten Pluton (Ukraine), reporting the presence of a large variation of different types of filaments in the studied organisms, and providing evidence of the presence of fungi-like organisms and continental deep biosphere by 1.5 billion years ago.[307]
- Hoshino et al. (2023) study the distribution of hopanoid C-2 methyltransferase in the bacterial domain, and interpret their findings as indicating that Alphaproteobacteria evolved hopanoid C-2 methyltransferase around 750 million years ago, thus re-establishing 2-methylhopanes as cyanobacterial biomarkers before 750 Ma.[308]
- Strullu-Derrien et al. (2023) describe new fossil material of nostocalean cyanobacteria from the Devonian Rhynie chert, interpret both new fossils and similar specimens that were already known (including fossils of Kidstoniella fritschii and Rhyniella vermiformis) as fossil material of a single species Langiella scourfieldii that belonged to the family Hapalosiphonaceae and thrived in soils, freshwater and hot springs like its extant relatives.[309]
- Li et al. (2023) describe new fossil material of Horodyskia from the Tonian Shiwangzhuang and Jiuliqiao formations (China), and reconstruct Horodyskia as a colonial organism composed of a chain of organic-walled vesicles that likely represent multinucleated cells of early eukaryotes.[310]
- Li et al. (2023) interpret discoidal fossils from the Tonian Jiuliqiao Formation (Anhui, China) as detached holdfasts of the worm-like annulated tubular fossils from the same formation.[311]
- Evidence of widespread presence of pyritized spherical microorganisms (likely coccoid bacterial body fossils) on the surface of invertebrate fossils from the Lower Cretaceous Crato Formation (Brazil) is presented by Barling, Saleh & Ma (2023).[312]
- Bryłka et al. (2023) reevaluate purported earliest fossils of diatoms from the Early and Middle Jurassic, and interpret them as unlikely to be fossil material of diatoms.[313]
- Evidence of the impact of nutrient availability gradient on changes in the calcareous dinocyst assemblages is reported from the Turonian Dubivtsi Formation (Ukraine) by Ciurej, Dubicka & Poberezhskyy (2023).[314]
- A study on the Cretaceous benthic foraminiferal assemblages from the Western Interior Seaway is published by Bryant, Meehan & Belanger (2023), who find no genera, guilds or morphotypes unique to cold seeps, and find assemblages from cold seeps to be overall more similar to offshore assemblages than nearshore ones, but also report that the composition of the studied assemblages did reflect the environmental differences present at seeps.[315]
- A study on the fossil record of the planktonic foraminifera, interpreted as indicating that a modern-style latitudinal diversity gradient for these foraminifera arose only 15 million years ago, is published by Fenton et al. (2023).[316]
- A study on the geographical distribution of the ecological and morphological groups of fossil planktonic foraminifera, interpreted as indicative of a global shift towards the Equator over the past 8 million years in response to the late Cenozoic temperature changes related to the polar ice sheet formation, is published by Woodhouse et al. (2023).[317]
- Fonseca et al. (2023) describe possible fossil material of choanoflagellates from the Upper Cretaceous (Cenomanian–Turonian) Capas Blancas Formation (Spain), representing the first putative occurrence of choanoflagellates in the fossil record reported to date.[318]
History of life in general
[edit]- Brocks et al. (2023) report the discovery of abundant protosteroids in sedimentary rocks of mid-Proterozoic age, and interpret this finding as evidence of the existence of a widespread and abundant biota of protosterol-producing bacteria and stem-group eukaryotes, living in aquatic environments from at least 1,640 to around 800 million years ago.[319]
- Choudhuri et al. (2023) describe exceptionally-preserved bedding plane structures from the 1.6-billion-years-old Chorhat Sandstone (India), and argue that some of the studied structures were more likely to be created by movement through a microbiota-rich surficial sediment than by passive migration of any inorganic or organic masses under influence of an external force.[320]
- Possible body and trace fossils, representing the oldest potential macrofossils from the Nama Group, are described from the lower Mara Member of the lower Dabis Formation (Tsaus Mountains, Namibia) by Wood et al. (2023), who interpret the studied fossils as remains of holdover soft-bodied taxa that appeared prior to the appearance of tubular and biomineralized animals.[321]
- A study on the timing and environmental context of the earliest biotic assemblage from the Nama Group, based on data from the Dabis Formation (Tsaus Mountains, Namibia), is published by Bowyer et al. (2023), who interpret their findings as indicating that the evolution of skeletonization and the first appearance of Cloudina happened in open marine carbonate settings and might have been driven by major sea level lowstands.[322]
- Kolesnikov et al. (2023) report the discovery of the fossil material of Ediacara-type soft-bodied organisms, including palaeopascichnids, arboreomorphs, chuariomorphids, microbial colonies, from the Dzhezhim Formation of the Timan Range (Komi Republic, Russia).[323]
- Revision of the Ediacaran fossils and pseudofossils from the Ura Formation (Patom Basin; Russia) is published by Petrov & Vorob'eva (2023).[324]
- Mussini & Dunn (2023) interpret the gradual but escalatory upping of ecological pressure resulting from evolutionary innovations such as bioturbation, predation and reef building as likely to be the most significant cause of the replacement of the Ediacaran biota by Phanerozoic biotas dominated by crown eumetazoans, and argue that changes to the originally homogenous distribution of resources in the benthos initiated by the Ediacaran biota itself might have driven the origins of bilaterians, their evolutionary innovations and ultimately their takeover.[325]
- Servais et al. (2023) review estimates of taxonomic richness of marine organisms during the early Paleozoic based on different published datasets, and question the existence of a distinct Cambrian explosion and global Ordovician biodiversification event instead of a single, long-term radiation of life during the early Paleozoic.[326]
- Evidence indicating that continental configuration and climate state specific to the early Paleozoic resulted in higher susceptibility of marine animals to extinction than during the rest of the Phanerozoic is presented by Pohl et al. (2023).[327]
- A study on the extinction selectivity of benthic brachiopods belonging to the groups Rhynchonellata and Strophomenata, gastropods, bivalves and trilobites throughout the Phanerozoic is published by Monarrez, Heim & Payne (2023), who report evidence of stronger extinction selectivity with respect to geographical range than body size, particularly during background intervals, but also evidence indicating that Phanerozoic mass extinctions may have been overall less selective than extinctions during background intervals, as well as indicative of more variable strength and direction of extinction selectivity by clade during Phanerozoic mass extinctions relative to background intervals.[328]
- Høyberget et al. (2023) report the discovery of a new, diverse early Cambrian biota (the Skyberg Biota) from the Skyberg Member of the Ringstranda Formation (Norway).[329]
- Li et al. (2023) compare the lamello-fibrillar nacre and similar fibrous microstructures in Early Cambrian molluscs and hyoliths from the Zavkhan Basin (Mongolia) and in extant coleoid cuttlebones and serpulid tubes, report differences in shell microstructures of the studied lophotrochozoan groups, and interpret their findings as indicative of prevalence of calcitic shells in the Terreneuvian.[330]
- A study aiming to identify the biases affecting the knowledge of the biodiversity during the Cambrian and Ordovician is published by Du et al. (2023), who interpret the significant decline in known biodiversity during Furongian interval as influenced by temporal, geographic, taxonomic and lithological biases, hindering the understanding of the real biodiversity changes in this interval.[331]
- Eliahou Ontiveros et al. (2023) study possible causes of the Great Ordovician Biodiversification Event, and interpret global cooling as the most likely primary driver.[332]
- A diverse Ordovician fauna (the Castle Bank fauna), comparable with the Burgess Shale and Chengjiang biotas in paleoenvironment and preservational style, is described from Wales (United Kingdom) by Botting et al. (2023).[333]
- A study on the structure of the Givetian shallow-water reef ecosystem from the Madène el Mrakib site (Morocco) is published by Majchrzyk et al. (2023), who report that the studied community from most known Devonian reefs, as it was dominated by large branching tabulate corals while stromatoporoids were of minor importance, and note similarities between the studied community and extant shallow-water reefs.[334]
- A study on the paleosols from the Devonian Zhongning Formation (China) is published by Guo, Retallack & Liu (2023), who find the paleosols and palaeobotany of the fossil bed where the fossil material of Sinostega was found to be similar to those of Devonian tetrapod localities in Pennsylvania, and interpret their findings as indicating that early tetrapods lived in meandering streams in semiarid to subhumid woodlands.[335]
- A study on the fossil record of tetrapods living from the Bashkirian to the Kungurian is published by Dunne et al. (2023), who argue that apparent changes in diversity of the studied tetrapods can be explained by variation in sampling intensity through time.[336]
- Francischini et al. (2023) describe straight, curved and quasi-helical burrows from the Permo-Triassic Buena Vista Formation (Brazil), similar to burrows reported from the Karoo Basin of South Africa, and interpret the studied burrows as likely produced by synapsids and/or procolophonians living in a desert environment, representing the oldest unambiguous record of tetrapod dwelling structures in such an environment.[337]
- A study on the impact of the Permian–Triassic extinction event on the marine ecosystems is published by Huang et al. (2023), who find that the first extinction phase resulted in the loss of more than half of taxonomic diversity but only a slight decrease of community stability, which subsequently decreased significantly in the second extinction phase.[338]
- Evidence indicating that reef recovery in the aftermath of the Permian–Triassic extinction was gradual and delayed compared to nonreef ecosystems is presented by Kelley et al. (2023).[339]
- Dai et al. (2023) report the discovery of an exceptionally preserved Early Triassic (approximately 250.8 million years ago) fossil assemblage (the Guiyang biota) from the Daye Formation near Guiyang (China), providing evidence of the existence of a complex marine ecosystem shortly after the Permian–Triassic extinction event.[340]
- Czepiński et al. (2023) report the discovery of a new, diverse vertebrate assemblage from the Ladinian Miedary site (Poland), including abundant fossil material of Tanystropheus, making the studied site the richest source of three-dimensionally preserved Tanystropheus material in the world reported to date.[341]
- New information on the composition of the Late Triassic paleocommunity from the Polzberg Lagerstätte (Austria), based on data from thousands of new fossils, is published by Lukeneder & Lukeneder (2023).[342]
- A study comparing changes in the marine and terrestrial biospheres across the Triassic-Jurassic transition is published by Cribb et al. (2023), who find evidence interpreted as suggestive of greater ecological severity of the Triassic–Jurassic extinction event for terrestrial ecosystems than marine ones.[343]
- El Atfy, Abeed & Uhl (2023) describe a diverse assemblage of non-pollen palynomorphs from the Lower Cretaceous (Berriasian-Valanginian) Yamama Formation (Iraq), and interpret the studied assemblage as deposited in anoxic, neritic conditions relatively near to the land.[344]
- Del Mouro et al. (2023) provide evidence of the preservation of organic walled microfossils (including pollen grains, spores and acritarchs) from wet peperites from the Lower Cretaceous Paraná-Etendeka intertrappean deposits of the Paraná Basin (Brazil), and interpret the studied microfossils as indicative of changes from desertic to more humid conditions in south-central Gondwana during the Valanginian.[345]
- Cortés & Larsson (2023) reconstruct the ecological network of the marine Mesozoic fauna from the Lower Cretaceous Paja Formation (Colombia), who report that the largest marine reptile predators from the studied fauna occupied higher trophic levels than any extant marine apex predator.[346]
- A study on the fossil record of Late Cretaceous invertebrates from the Western Interior Seaway and the adjacent Gulf Coastal Plain is published by Purcell, Scuderi & Myers (2023), who interpret their findings as indicating that the Western Interior Seaway did not contain biotic subprovinces in the Late Cretaceous, but faunal associations were affected by sea-level changes.[347]
- Description of a diverse Santonian-?early Campanian marine vertebrate assemblage from the Akkermanovka locality (Orenburg Oblast, Russia), including fossil material of a mosasaur, plesiosaurs, bony and cartilaginous fishes (with lamniform sharks being the most diverse and abundant group in the assemblage), is published by Jambura et al. (2023).[348]
- Bobe et al. (2023) describe fossil material of marine and terrestrial animals (including a new hyrax taxon) and woods from new sites from the Miocene Mazamba Formation (Mozambique), and interpret the studied sites as formed in coastal settings.[349]
- Hayward et al. (2023) report the discovery of a diverse Pliocene (Waipipian) fauna from sediment excavated from two shafts at Mangere Wastewater Treatment Plant (New Zealand), dominated by molluscs and including new species records for New Zealand, as well as extending known time ranges of taxa already known from New Zealand.[350]
- Harrison et al. (2023) provide the systematic account of the Pliocene fauna from the Lower Laetolil Beds (Laetoli, Tanzania).[351]
- A study on the timing of Pleistocene megafaunal extinction in the high plains of Peru is published by Rozas-Davila, Rodbell & Bush (2023), who find that the collapse of megafaunal populations in high grasslands coincided with upticks in fire activity, which were likely associated with human activity.[352]
- Martinez et al. (2023) find no evidence of a significant relation between the relative surface area of the maxilloturbinal and physiological traits such as metabolism and body temperature in extant mammals, and interpret their findings as challenging the hypothesis positing that respiratory turbinals reflect the thermal and metabolic physiology in extant and extinct tetrapods (especially in mammals).[353]
Other research
[edit]- A study on changes to the regional and global geochemical environment in the aftermath of the Sturtian glaciation is published by Bowyer et al. (2023), who find that the shift to dominant green algal primary production and the first appearance of putative sponges and problematic macrofossils might be related to global stabilization of geochemical environments following the deglaciation, to the expansion of less reducing (and likely more oligotrophic) marine environments, and to the shift from postglacial super-greenhouse conditions to a cooler climate.[354]
- Evidence from the Cryogenian Nantuo Formation (China), interpreted as indicating that habitable open ocean conditions providing refugia for eukaryotic organisms during the Marinoan glaciation extended into mid-latitude coastal oceans, is presented by Song et al. (2023).[355]
- Evidence of the impact of tectonic and ecological factors on redox changes in upper ocean, deep shelf and restricted basin settings throughout the Phanerozoic, which in turn were correlated with background extinction rates of marine animals, is presented by Wang et al. (2023).[356]
- A study on the stratigraphy of the Siberian Platform (Russia), and on its implications for the knowledge of the age of the fossils and timing of first appearances of late Ediacaran and early Cambrian organisms from the Siberian Platform (including anabaritids and cloudinids), is published by Bowyer et al. (2023).[357]
- A study on the evolution of the Earth's landscape throughouth the Phanerozoic, providing evidence of impact of landscape dynamics on the diversification of both marine life and terrestrial life, is published by Salles et al. (2023).[358]
- Nelson et al. (2023) present high-precision age constraints for the lower Wood Canyon Formation (Nevada, United States), and interpret their findings as indicating that the base of the Cambrian Period was younger than 533 million years ago, making the early Cambrian animal radiation faster than previously recognized.[359]
- Nolan et al. (2023) interpret Brooksella alternata as a likely pseudofossil, and the bulk of its characteristics as consistent with concretions.[360]
- Wellman et al. (2023) present data supporting a Silurian (late Wenlock) age of the "Lower Old Red Sandstone" deposits of the Midland Valley (Scotland, United Kingdom) preserving the fossil material of Pneumodesmus newmani, supporting the interpretation of this myriapod as the oldest known air-breathing land animal.[361]
- A study on the preservation of chemical information in the fossils from the Devonian Rhynie chert (United Kingdom) is published by Loron et al. (2023), who report that differences between prokaryotes and eukaryotes and between eukaryotic tissue types from the Rhynie chert assemblage can be identified based on the fossilization products of lipids, sugar and protein.[362]
- A study on the geochemistry of the Bakken Formation, interpreted as indicative of stepwise transgressions of toxic euxinic waters into the shallow oceans that drove a series of Late Devonian extinction events, is published by Sahoo et al. (2023).[363]
- Evidence from mercury concentrations and isotopes from terrestrial sections from the Sydney Basin (Australia) and Karoo Basin (South Africa), interpreted as indicative of global volcanic effects of the Siberian Traps during the Permian-Triassic transition, is presented by Shen et al. (2023).[364]
- Evidence from concentrations of UV-B–absorbing compounds in the exine of fossil pollen from the Qubu section in southern Tibet (China), interpreted as consistent with increased UV-B radiation during the Permian–Triassic extinction event, is presented by Liu et al. (2023);[365] their conclusions are subsequently contested by Seddon & Zimmermann (2023).[366][367]
- Lovelace et al. (2023) present data supporting a Carnian age for the majority of the Popo Agie Formation.[368]
- Evidence from molybdenum records from cores in Germany and Northern Ireland corresponding to the Tethyan shelf, interpreted as indicative of pulses of localized marine de-oxygenation which were limited largely to marginal marine environments and likely related to shallow-marine extinctions at the end of the Triassic, is presented by Bond et al. (2023).[369]
- Sedimentologic evidence of glaciers developing in continental Iberia during the Hauterivian is reported from the Enciso Group in the eastern Cameros Basin (Spain) by Rodríguez-López et al. (2023).[370]
- A study on the Cenomanian–Turonian benthic foraminiferal assemblages from the Western Interior Seaway is published by Bryant & Belanger (2023), who report that the interval of increased density and diversity of benthic foraminifera known as the Benthonic Zone is not a reliable biostratigraphic marker for the onset of the Oceanic Anoxic Event 2 in the Western Interior Seaway, and that different samples of the Benthonic Zone don't reflect basin-wide changes in oxygenation.[371]
- Evidence from two sites offshore of southwest Australia, interpreted as indicative of ocean acidification at the onset of Oceanic Anoxic Event 2 which was linked to the onset of volcanic activity, and which persisted for approximately 600,000 years due to biogeochemical feedbacks, is presented by Jones et al. (2023).[372]
- Evidence from concentrations of sulfur and fluorine in Deccan Traps lavas, interpreted as indicative of recurring eruptive pulses of Deccan Traps volcanism before the Cretaceous–Paleogene extinction event which might have caused short-lived global drops in temperature, is presented by Callegaro et al. (2023).[373]
- A study on the history of the Eocene waterbody within the Giraffe Pipe crater (Northwest Territories, Canada), inferred from changes in the fossil record of microorganisms, is published by Siver & Lott (2023), who interpret their findings as indicative of the presence of a series of successive shallow environments, each correlated with changes in lakewater chemistry.[374]
- Evidence from tooth enamel of specimens of Hippopotamus antiquus from Early Pleistocene sites in Southern and Central Europe (primarily from Upper Valdarno and Vallparadís Section in Italy and Spain, respectively), interpreted as indicative of a progressive increase of environmental seasonality around the Mediterranean Basin during the Early Pleistocene, is presented by Fidalgo et al. (2023).[375]
- Abbas et al. (2023) report the presence of Late Quaternary wetland sediments at the Wadi Hasa, Gregra and Wadi Gharandal areas in the Jordan desert, and interpret their findings as indicating that during the Marine Isotope Stage 5 the Levant was a well-watered route for human dispersal out of Africa.[376]
- Essel et al. (2023) report the development of a new method for the gradual release of DNA trapped in ancient bone and tooth artefacts, and use this method to recover ancient human and deer mitochondrial genomes from the Upper Paleolithic deer tooth pendant from Denisova Cave (Russia).[377]
- Reeves & Sansom (2023) present a new method which can be used to determine the impact of multiple factors (decay, ontogeny and phylogeny) on morphological variation between fossils, and apply this method to fossils of Tethymyxine, Mayomyzon, Priscomyzon, "euphaneropoids" and Palaeospondylus.[378]
- Wang, Shu & Wang (2023) present a new method for element mapping of the fossils' 3D surface using nondestructive X-ray fluorescence, providing information on degraded material from the soft body parts in the sediments surrounding the studied fossil specimens, and apply this method to a specimen of Keichousaurus hui.[379]
- Slater et al. (2023) study the impact of thermal maturation on eumelanin and phaeomelanin, develop a predictive model for authentic signals for eumelanin and phaeomelanin in fossil tissues, and use this model to provide molecular evidence of preservation of eumelanin in fossil feathers of Confuciusornis, as well as molecular evidence of preservation of phaeomelanin in the fossil material of the Miocene frog Pelophylax pueyoi.[380]
- Peters et al. (2023) study collagen survival in bones from Quaternary sites across Australia, providing evidence of preservation of bone collagen dating back more than 50,000 years in the material from Tripot Cave in the subtropical Broken River limestone karst area.[381]
- Brooke et al. (2023) demonstrate the utility of agent-based modelling for study of the ecologies of past ecosystems, using such a model to determine the drivers of distribution of large ungulates from the Palaeo-Agulhas Plain during the peak of the Last Glacial Maximum.[382]
Paleoclimate
[edit]- A study on the evolution of the monsoon system over the past 250 million years, providing evidence of the impact of continental area, latitudinal location and fragmentation, is published by Hu et al. (2023).[383]
- Evidence indicating that injection of the silicate dust from the Chicxulub impact into the atmosphere contributed to the global cooling and disruption of photosynthesis that followed is presented by Senel et al. (2023).[384]
- The Cenozoic CO2 Proxy Integration Project (CenCO2PIP) Consortium reconstructs the Cenozoic history of atmospheric CO2 on the basis of reevaluation of all available proxies.[385]
- Evidence from seawater osmium isotope data from Pacific Ocean sediments, interpreted as indicating that enhanced magmatism could have played a dominant role in causing the Miocene Climatic Optimum, is presented by Goto et al. (2023).[386]
- Wen et al. (2023) present a new land surface temperature record from the Chinese Loess Plateau in East Asia, interpreting it as indicative of late Miocene cooling and aridification that occurred synchronously with ocean cooling, highlighting a global climate forcing mechanism.[387]
- Evidence of periodic deposition of sapropelic mud in Eastern Mediterranean during the Plio-Pleistocene and records of precipitation and vegetation from leaf wax biomarkers from the studied sapropel layers, interpreted as indicative of the impact of orbital cycles on monsoon variability in northeast Africa and on greening of the Sahara which created routes of dispersal out of Africa for hominins, is presented by Lupien et al. (2023).[388]
- Margari et al. (2023) provide evidence of pronounced climate variability in Europe during a glacial period ~1.154 to ~1.123 million years ago, culminating in extreme glacial cooling, and argue that these conditions led to the depopulation of Europe.[389]
References
[edit]- ^ Gini-Newman, Garfield; Graham, Elizabeth (2001). Echoes from the past: world history to the 16th century. Toronto: McGraw-Hill Ryerson Ltd. ISBN 9780070887398. OCLC 46769716.
- ^ a b c Qin, S.; Dong, L.; Liu, X.; Tang, F.; Liu, H. (2023). "Macroscopic carbonaceous compression fossils from the Tonian Liulaobei Formation in the Huainan region of North China". Precambrian Research. 393. 107076. Bibcode:2023PreR..39307076Q. doi:10.1016/j.precamres.2023.107076. S2CID 258997321.
- ^ a b c Liu, J.; Zhang, Y.; Shi, X.; Chen, A.; Tang, D.; Yang, T. (2023). "Macroscopic fossils from the Chuanlinggou Formation of North China: evidence for an earlier origin of multicellular algae in the late Palaeoproterozoic". Palaeontology. 66 (6). e12685. Bibcode:2023Palgy..6612685L. doi:10.1111/pala.12685. S2CID 266443713.
- ^ Liu, J.; Li, M.; Tang, F.; Zhao, J.; Song, S.; Zhou, Y.; Song, X.; Ren, L. (2023). "New Benthic Fossils from the Late Ediacaran Strata of Southwestern China". Acta Geologica Sinica (English Edition). 98 (2): 311–323. doi:10.1111/1755-6724.15153.
- ^ Grasby, S. E.; Ardakani, O. H.; Liu, X.; Bond, D. P. G.; Wignall, P. B.; Strachan, L. J. (2023). "Marine snowstorm during the Permian−Triassic mass extinction". Geology. 52 (2): 120–124. doi:10.1130/G51497.1.
- ^ Dey, R.; Basso, D.; Chakraborty, A.; Roy, L.; Bhaumik, A. K.; Ghosh, A. K. (2023). "Rhodolith-forming coralline red algae in the CaCO3 biofactory — A case study from the Serravallian of tropical northeastern Indian Ocean". Comptes Rendus Palevol. 22 (26): 541–567. doi:10.5852/cr-palevol2023v22a26. hdl:10281/436018. S2CID 261008390.
- ^ Kundu, S.; Khan, M. A. (2023). "Black mildew disease on the Siwalik (Miocene) monocot leaves of Western Himalaya, India caused by Meliolinites". Fungal Biology. 128 (1): 1626–1637. doi:10.1016/j.funbio.2023.12.006. PMID 38341268.
- ^ Retallack, G. J. (2023). "Why was there a Neoproterozoic Snowball Earth?". Precambrian Research. 385. 106952. Bibcode:2023PreR..38506952R. doi:10.1016/j.precamres.2022.106952. S2CID 255647705.
- ^ Strullu-Derrien, C.; Góral, T.; Spencer, A. R. T.; Kenrick, P.; Aime, M. C.; Gaya, E.; Hawksworth, D. L. (2023). "A fungal plant pathogen discovered in the Devonian Rhynie Chert". Nature Communications. 14 (1). 7932. Bibcode:2023NatCo..14.7932S. doi:10.1038/s41467-023-43276-1. PMC 10692235. PMID 38040707.
- ^ Krings, M.; Harper, C. J. (2023). "A fungal mycelium containing abundant endoconidia from the Lower Devonian Rhynie cherts of Scotland". Review of Palaeobotany and Palynology. 313. 104891. Bibcode:2023RPaPa.31304891K. doi:10.1016/j.revpalbo.2023.104891. S2CID 257907523.
- ^ Worobiec, G.; Piątek, M.; Worobiec, E. (2023). "Szaferomyces pliocenicus nov. gen., nov. sp. from the upper Pliocene deposits of Mizerna (Poland), a fossil fungus showing close resemblance to modern powdery mildews". Geobios. 79: 77–82. Bibcode:2023Geobi..79...77W. doi:10.1016/j.geobios.2023.05.006. S2CID 259941219.
- ^ Krings, M.; Harper, C. J.; Decombeix, A.-L.; Galtier, J. (2023). "The core of Sporocarpon asteroides, an enigmatic fungal fossil from the Carboniferous" (PDF). Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 309 (2): 111–122. doi:10.1127/njgpa/2023/1153. S2CID 261596545.
- ^ Sukhomlyn, M. M.; Perkovsky, E. E. (2023). "First carnivorous fungus from Santonian Taimyr amber". Earth and Environmental Science Transactions of the Royal Society of Edinburgh. 114 (1–2): 183–188. Bibcode:2023EESTR.114..183S. doi:10.1017/S1755691023000087. S2CID 259408576.
- ^ Worobiec, G.; Worobiec, E.; Liu, Y. (C.) (2023). "Taxonomy and palaeoecology of the fossil anamorphic fungus Mycoenterolobium eccentricum (R.K. Kar) G. Worobiec, n. comb". Comptes Rendus Palevol. 22 (28): 585–594. doi:10.5852/cr-palevol2023v22a28. S2CID 261564702.
- ^ Niko, S.; Suzuki, S. (2023). "Miocene scleractinian corals from the Kennon Limestone of the Province of Benguet, Luzon Island, northern Philippines". Bulletin of the Akiyoshi-dai Museum of Natural History. 58: 11–15.
- ^ a b c d El-Desouky, H.; Herbig, H.-G.; Kora, M. (2023). "Kasimovian (late Pennsylvanian) cornute rugose corals from Egypt: taxonomy, facies and palaeogeography of a cool-water fauna from northern Gondwana". Swiss Journal of Palaeontology. 142 (1). 32. Bibcode:2023SwJP..142...32E. doi:10.1186/s13358-023-00296-0.
- ^ a b c Samaniego-Pesqueira, A.; Löser, H.; Moreno-Bedmar, J. A. (2023). "Middle Albian corals from the Espinazo del Diablo Formation (Lampazos area, Sonora, Mexico)". Bulletin of Geosciences. 98 (2): 111–159. doi:10.3140/bull.geosci.1872. S2CID 259636431.
- ^ Larson, E.; Briggs, D. E. G. (2023). "A hydrozoan from the eurypterid-dominated Silurian Bertie Group Lagerstätten of North America". Journal of Paleontology. 97 (5): 1002–1008. Bibcode:2023JPal...97.1002L. doi:10.1017/jpa.2023.62.
- ^ Moon, J.; Caron, J.-B.; Moysiuk, J. (2023). "A macroscopic free-swimming medusa from the middle Cambrian Burgess Shale". Proceedings of the Royal Society B: Biological Sciences. 290 (2004). 20222490. doi:10.1098/rspb.2022.2490. PMC 10394413. PMID 37528711.
- ^ Niko, S. (2023). "Late Silurian halysitid corals from the Okanaro Group in Ehime Prefecture, Southwest Japan". Bulletin of the National Museum of Nature and Science, Series C. 49: 73–79. doi:10.50826/bnmnsgeopaleo.49.0_73.
- ^ Song, Z.; Guo, J.; Han, J.; Van Iten, H.; Qiang, Y.; Peng, J.; Sun, J.; Zheng, Y.; Huang, X.; Zhang, Z. (2023). "A new species of Decimoconularia (Cnidaria, Medusozoa) from the Lower Cambrian of South China". Frontiers in Earth Science. 10. 1048800. Bibcode:2023FrEaS..1048800S. doi:10.3389/feart.2022.1048800.
- ^ Löser, H.; Werner, W.; Darga, R. (2023). "Middle Cenomanian coral fauna from the Roßsteinalmen (Northern Calcareous Alps, Bavaria, Southern Germany) – a revised and extended version". Zitteliana. 97: 89–147. doi:10.3897/zitteliana.97.113796.
- ^ Niko, S. (2023). "Middle Devonian auloporid corals from the Naidaijin Formation, Kumamoto Prefecture, Southwest Japan". Bulletin of the National Museum of Nature and Science, Series C. 49: 67–71. doi:10.50826/bnmnsgeopaleo.49.0_67.
- ^ Niko, S. (2023). "Notocyathus suzukii, a new Miocene species of scieractinian coral from the Katsuta Group in the Tsuyama area, Okayama Prefecture, Southwest Japan". Bulletin of the Akiyoshi-dai Museum of Natural History. 58: 7–10.
- ^ Qu, H.; Li, K.; Ou, Q. (2023). "Thecate stem medusozoans (Cnidaria) from the early Cambrian Chengjiang biota". Palaeontology. 66 (1). e12636. Bibcode:2023Palgy..6612636Q. doi:10.1111/pala.12636. S2CID 256562444.
- ^ a b c d Mergl, M.; Kraft, P. (2023). "Byronids and similar tubular fossils from the Devonian of the Barrandian area (Czech Republic)". Earth and Environmental Science Transactions of the Royal Society of Edinburgh. 113 (4): 373–390. doi:10.1017/S1755691023000099. S2CID 258973908.
- ^ Lathuilière, B.; Schlögl, J.; Tomašových, A.; Ivanova, D. K. (2023). "Coral assemblages and environments from Bajocian reefs in the Western Carpathians". Geobios. 79: 17–41. Bibcode:2023Geobi..79...17L. doi:10.1016/j.geobios.2023.06.001. S2CID 260007930.
- ^ Guedes, C. B.; Siviero, F.; Scheffler, S. M. (2023). "Taxonomy of Devonian conulariids (Cnidaria) from Mato Grosso do Sul, Paraná Basin, Brazil". Revista Brasileira de Paleontologia. 26 (1): 3–12. doi:10.4072/rbp.2023.1.01. S2CID 259526214.
- ^ Hachour, K.; Hamdidouche, R.; Dahoumane, A.; Goucem, A. (2023). "A new triradial species from the Neoproterozoic formations of the Chenachene region, north-east of the Taoudeni basin (south-western Algeria)". Journal of African Earth Sciences. 202. 104934. Bibcode:2023JAfES.20204934H. doi:10.1016/j.jafrearsci.2023.104934. S2CID 258069573.
- ^ Elias, R. J.; Hewitt, R. A. (2023). "Corals and a cephalopod from the Whirlpool Formation (latest Ordovician, Hirnantian), Hamilton, Ontario: biostratigraphic and biogeographic significance". Journal of Paleontology. 97 (4): 805–822. Bibcode:2023JPal...97..805E. doi:10.1017/jpa.2023.53.
- ^ Niko, S. (2023). "Stylina eguchii, a new Aptian (Early Cretaceous) species of scleractinian coral from the Miyako Group in the Hideshima coastal area, Iwate Prefecture, Northeast Japan". Bulletin of the Akiyoshi-dai Museum of Natural History. 58: 1–5.
- ^ Reich, M.; Kutscher, M. (2023). "A new alcyonacean octocoral (Anthozoa) from the Late Silurian of Gotland, Sweden". PalZ. 97 (4): 729–739. Bibcode:2023PalZ...97..729R. doi:10.1007/s12542-023-00654-w. S2CID 259775382.
- ^ Hageman, S. J.; Vinn, O. (2023). "Late Cambrian Pywackia is a cnidarian, not a bryozoan: Insights from skeletal microstructure". Journal of Paleontology. 97 (5): 990–1001. Bibcode:2023JPal...97..990H. doi:10.1017/jpa.2023.35. S2CID 259608822.
- ^ Sendino, C.; Clark, B.; Morandini, A. C.; Salge, T.; Lowe, M.; Rushlau, W. (2023). "Internal conulariid structures unveiled using µCT". PalZ. 97 (3): 451–467. Bibcode:2023PalZ...97..451S. doi:10.1007/s12542-023-00649-7. S2CID 257873488.
- ^ Van Iten, H.; Hughes, N. C; John, D. L.; Gaines, R. R.; Colbert, M. W. (2023). "Conulariid soft parts replicated in silica from the Scotch Grove Formation (lower Middle Silurian) of east-central Iowa". Journal of Paleontology. 97 (5): 961–970. Bibcode:2023JPal...97..961V. doi:10.1017/jpa.2023.6. S2CID 258389436.
- ^ Zhao, Y.; Parry, L. A.; Vinther, J.; Dunn, F. S.; Li, Y.-J.; Wei, F.; Hou, X.-G.; Cong, P.-Y. (2023). "An early Cambrian polyp reveals a potential anemone-like ancestor for medusozoan cnidarians". Palaeontology. 66 (1). e12637. Bibcode:2023Palgy..6612637Z. doi:10.1111/pala.12637. S2CID 256752700.
- ^ Zhang, Y.; Liu, Y.; Shao, T.; Qin, J. (2023). "New Qinscyphus material from the Fortunian of South China". Frontiers in Earth Science. 11. 1038686. Bibcode:2023FrEaS..1138686Z. doi:10.3389/feart.2023.1038686.
- ^ Plotnick, R. E.; Young, G. A.; Hagadorn, J. W. (2023). "An abundant sea anemone from the Carboniferous Mazon Creek Lagerstӓtte, USA". Papers in Palaeontology. 9 (2). e1479. Bibcode:2023PPal....9E1479P. doi:10.1002/spp2.1479. S2CID 257447889.
- ^ a b c d e f Ernst, A.; Rodríguez, S. (2023). "Palaeoecology and palaeobiogeographic relationships of Lower Devonian bryozoans from the Guadámez and Peñón Cortado Sections of Sierra Morena (SW Spain)". Spanish Journal of Palaeontology. 38 (2): 173–220. doi:10.7203/sjp.26580. hdl:10261/359231. S2CID 258847830.
- ^ a b Koromyslova, A. V. (2023). "New cyclostome bryozoans from the Lower Cretaceous of Dagestan". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 309 (1): 49–64. doi:10.1127/njgpa/2023/1148. S2CID 260595425.
- ^ a b Arakawa, S. (2022). "Iodictyum akaishiensis sp. nov.: A New Miocene Phidoloporid (Bryozoa, Cheilostomata) from the Moniwa Formation, Sendai, Japan". Paleontological Research. 27 (1): 25–33. doi:10.2517/PR200041. S2CID 252684206.
- ^ López-Gappa, J.; Pérez, L. M. (2023). "Species of the genus Melicerita Milne Edwards (Bryozoa, Cheilostomatida) in the Early Miocene of Patagonia (Argentina)". Publicación Electrónica de la Asociación Paleontológica Argentina. 23 (1): 49–60. doi:10.5710/PEAPA.15.02.2023.448. S2CID 257933615.
- ^ a b c d Arakawa, S. (2023). "Five Species of Microporina (Bryozoa, Cheilostomata) from the Pleistocene Setana Formation at Kuromatsunai, Hokkaido, Japan". Paleontological Research. 27 (3): 245–260. doi:10.2517/PR210017. S2CID 255441175.
- ^ Tolokonnikova, Z. A.; Fedorov, P. V. (2023). "Morphological features of Late Ordovician (Sandbian) bryozoans from the basin of Khrevitsa River (north-western Russia) and description of a new species of the genus Prophyllodictya Gorjunova, 1987". Zootaxa. 5284 (2): 337–350. doi:10.11646/zootaxa.5284.2.6. PMID 37518734. S2CID 258658525.
- ^ Pacaud, J.-M. (2023). "Spiropora flaviae nom. nov., un nouveau nom de remplacement pour Spiropora elegans Millet de la Turtaudière, 1865, non Lamouroux, 1821 (Bryozoa : Cyclostomata : Spiroporidae)". Fossiles. Revue française de paléontologie. 55: 53.
- ^ Ernst, A.; Tolokonnikova, Z. (2023). "Unusual cystoporate? bryozoan from the Upper Ordovician of Siljan District, Dalarna, central Sweden". GFF. 144 (3–4): 210–219. doi:10.1080/11035897.2023.2223579. S2CID 260402785.
- ^ Ernst, A. (2023). "New trepostome bryozoan genus from the Estonian Kukersite". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 307 (3): 249–259. doi:10.1127/njgpa/2023/1124. S2CID 258338595.
- ^ a b c Wang, Y.; Zhan, R.-B.; Luan, X.-C.; Zhang, Y.-C.; Wei, X. (2023). "Middle–Late Ordovician brachiopods from Ningnan, southern Sichuan Province, Southwest China: Implications for macroevolution and palaeogeography". Palaeoworld. 32 (2): 235–251. Bibcode:2023Palae..32..235W. doi:10.1016/j.palwor.2023.02.007. S2CID 257372057.
- ^ Mergl, M.; Šmídtová, N. (2023). "Lingulate brachiopods from the Vinařice Limestone (Devonian, Pragian) of the Barrandian area, Czechia". Bulletin of Geosciences. 98 (3): 199–214. doi:10.3140/bull.geosci.1880.
- ^ Serobyan, V.; Danelian, T.; Hairapetian, V.; Grigoryan, A.; Crônier, C.; Randon, C.; Mottequin, B. (2023). "Frasnian (Upper Devonian) brachiopods from Armenia: biostratigraphic and palaeobiogeographic implications". Rivista Italiana di Paleontologia e Stratigrafia. 129 (2): 373–409. doi:10.54103/2039-4942/19826. S2CID 259521043.
- ^ a b Bitner, M. A.; Bahrami, A.; Yazdi, M.; Zágoršek, K. (2023). "Brachiopods from the Lower Red Formation (Lower Oligocene) of the Isfahan Province, Central Iran" (PDF). Annales Societatis Geologorum Poloniae. 93 (4): 411–422. doi:10.14241/asgp.2023.17.
- ^ a b Radulović, B. V.; Metodiev, L. S.; Motchurova-Dekova, N.; Tchoumatchenco, P. (2023). "Early–Middle Jurassic brachiopods from Ponor Mountain, Western Balkan Mountains, Bulgaria; taxonomy, biostratigraphy and occurrence in the context of the early Toarcian Oceanic Anoxic Event". Historical Biology: An International Journal of Paleobiology. 36 (4): 829–856. doi:10.1080/08912963.2023.2190757. S2CID 258475418.
- ^ Lavié, F. J.; Benedetto, J. L. (2023). "Lower Tremadocian (Ordovician) lingulate brachiopods from the Central Andean Basin (NW Argentina) and their biogeographical links". Bulletin of Geosciences. 98 (1): 79–93. doi:10.3140/bull.geosci.1866. hdl:11336/226215. S2CID 258085439.
- ^ a b c MacFarlan, D. A. B. (2023). "Latest Triassic and Early Jurassic Spiriferinida (Brachiopoda) of Zealandia (New Zealand and New Caledonia)". Zootaxa. 5277 (1): 1–58. doi:10.11646/zootaxa.5277.1.1. PMID 37518332. S2CID 258442187.
- ^ Baranov, V. V.; Nasiri, Y.; Sharifi, J.; Blodgett, R. B.; Gharaie, M. H. M.; Taghdisi Nikbakht, S.; Hadi, M. (2023). "The First Finding of a Representative of the Genus Crinisarina Cooper and Dutro, 1982 (Athyridida) in the Late Famennian of Northeastern Iran". Paleontological Journal. 57 (7): 744–753. Bibcode:2023PalJ...57..744B. doi:10.1134/S003103012307002X. S2CID 265501158.
- ^ a b Baeza-Carratalá, J. F.; Berrocal-Casero, M.; García Joral, F. (2023). "Brachiopods from the Albian–Cenomanian transition (Cretaceous) of the Eastern Prebetic (South-Iberian paleomargin)". Cretaceous Research. 150. 105583. Bibcode:2023CrRes.15005583B. doi:10.1016/j.cretres.2023.105583. hdl:10045/134904. S2CID 259007270.
- ^ Berrocal-Casero, M.; Baeza-Carratalá, J. F.; García Joral, F. (2023). "A new asymmetric rhynchonellide from the Cretaceous of the Eastern Prebetic (Southeastern Spain)". Spanish Journal of Palaeontology. 38 (2): 221–234. doi:10.7203/sjp.26294. hdl:10045/133324. S2CID 257830348.
- ^ a b Pérez, D. E.; Farroni, N. D.; Allende Mosquera, A.; Cuitiño, J. I. (2023). "First discinid brachiopods (Brachiopoda: Lingulida) from the Cenozoic of Patagonia (Gaiman Formation, Lower Miocene, Argentina)". Ameghiniana. 60 (3): 203–215. doi:10.5710/AMGH.23.01.2023.3544. S2CID 256258549.
- ^ a b Zhang, Zhiliang; Zhang, Zhifei; Holmer, L. E.; Topper, T. P.; Pan, B.; Li, G. (2023). "Evolution and diversity of biomineralized columnar architecture in early Cambrian phosphatic-shelled brachiopods". eLife. 12. RP88855. doi:10.7554/eLife.88855. PMC 11006422. PMID 38597930.
- ^ a b Harper, D. A. T.; Bates, D. E. B. (2023). "Middle Ordovician brachiopods from Tagoat, Co. Wexford, SE Ireland: Dapingian diversity drivers". Geobios. 81: 85–100. Bibcode:2023Geobi..81...85H. doi:10.1016/j.geobios.2023.06.006. S2CID 260716289.
- ^ Waterhouse, J. B. (2023). "Permian species of Lingulida and Strophomeniformii from east Australia and New Zealand". A summary of Chonetidina (Brachiopoda) from the Permian faunas of east Australia and New Zealand (PDF). Earthwise. Vol. 21. pp. 37–64.
- ^ Tazawa, J.; Waterhouse, J. B. (2023). "A new paucispiniferoid brachiopod genus from the Permian of Japan". In J. B. Waterhouse (ed.). A summary of brachiopod species belonging to Lingulida, Strophomenida, Chonetidina and Productidina from the Permian faunas of East Australia and New Zealand (PDF). Earthwise. Vol. 21. pp. 227–235.
- ^ a b c Wu, H.; Zhang, Y.; Chen, A.; Stubbs, T. L. (2023). "A Highly Diverse Olenekian Brachiopod Fauna from the Nanpanjiang Basin, South China, and Its Implications for the Early Triassic Biotic Recovery". Biology. 12 (4). 622. doi:10.3390/biology12040622. PMC 10136273. PMID 37106822.
- ^ Oh, Y.; Lee, S.; Park, T.-Y. S.; Lee, D.-C. (2023). "Middle Ordovician (middle Darriwilian) Dirafinesquina and Jigunsania gen. nov. (Rafinesquinidae; Strophomenoidea; Brachiopoda) from South Korea, with discussion of rafinesquinid evolution". Geosciences Journal. 27 (3): 253–270. Bibcode:2023GescJ..27..253O. doi:10.1007/s12303-022-0034-x. S2CID 257047216.
- ^ a b c Baliński, A.; Halamski, A. T. (2023). "Pre-Taghanic (Lower to lower Middle Givetian) brachiopods from Miłoszów in the Holy Cross Mountains (Poland)". Annales Societatis Geologorum Poloniae. 93 (1): 3–102. doi:10.14241/asgp.2023.01.
- ^ a b c d e MacFarlan, D. A. B. (2023). "Otapirian (Rhaetian) Terebratulida (Brachiopoda) of Zealandia". Zootaxa. 5374 (1): 1–34. doi:10.11646/zootaxa.5374.1.1. PMID 38220875.
- ^ Baranov, V. V.; Nasiri, Y.; Gharaie, M. H. M.; Taghdisi Nikbakht, S.; Hadi, M. (2023). "A new species Martinothyris pseudolineatus (Spiriferida) from the Late Famennian of northeastern Iran". Paleontological Journal. 57 (6): 623–628. Bibcode:2023PalJ...57..623B. doi:10.1134/S0031030123040032. S2CID 265501175.
- ^ Waterhouse, J. B. (2023). "Permian species of Lingulida and Strophomeniformii from east Australia and New Zealand". A summary of brachiopod species belonging to Lingulida, Strophomenida, Chonetidina and Productidina from the Permian faunas of East Australia and New Zealand (PDF). Earthwise. Vol. 21. pp. 9–35.
- ^ a b c Waterhouse, J. B. (2023). "Permian Paucispinauriidae (Brachiopoda, suborder Linoproductidina) in east Australia and New Zealand, with mention of allies further afield". A summary of Chonetidina (Brachiopoda) from the Permian faunas of east Australia and New Zealand (PDF). Earthwise. Vol. 21. pp. 109–226.
- ^ a b c Wang, Y.N.; Ma, X.P.; Ebbighausen, V.; Becker, R. T. (2023). "Spiriferide brachiopods from the early Famennian (Late Devonian) of western Europe". Acta Palaeontologica Sinica. 62 (3): 350–375. doi:10.19800/j.cnki.aps.2022030.
- ^ a b c Baranov, V. V.; Blodgett, R. B. (2023). "Some Early Pragian Brachiopods from Soda Creek Limestone of West-Central Alaska". Paleontological Journal. 57 (1 supplement): S45–S57. doi:10.1134/S0031030123700016.
- ^ Jahangir, H.; Zhang, Z.; Popov, L. E.; Holmer, L. E.; Ghobadi Pour, M.; Zhan, R. (2023). "The siphonotretide brachiopod Schizambon from the Early Ordovician of South China: ontogeny and affinity". Papers in Palaeontology. 9 (4). e1517. Bibcode:2023PPal....9E1517J. doi:10.1002/spp2.1517. S2CID 260392889.
- ^ Feldman, H. R.; Blodgett, R. B.; Wilson, M. A.; Radulović, B. V. (2023). "Notes on some Jurassic (Callovian) brachiopods from Hamakhtesh Hagadol, southern Israel". New Mexico Museum of Natural History and Science Bulletin. 94: 219–226.
- ^ Lee, S.; Shi, G. R.; Runnegar, B.; Waterhouse, J. B. (2023). "Kungurian (Cisuralian/Early Permian) brachiopods from the Snapper Point Formation, southern Sydney Basin, southeastern Australia". Alcheringa: An Australasian Journal of Palaeontology. 47 (1): 67–108. Bibcode:2023Alch...47...67L. doi:10.1080/03115518.2022.2151045. S2CID 256076332.
- ^ Wenndorf, K.-W. (2023). "Neue Erkenntnisse zur Rhynchonelliden-Gattung Xahetomus (Brachiopoda) aus dem Unterdevon der Mittelrhein-Region". Mainzer geowissenschaftliche Mitteilungen. 51: 193–222. doi:10.23689/fidgeo-5828.
- ^ Paz, M.; Mángano, M. G.; Buatois, L. A.; Campetella, D. M.; Sproat, C.; Pérez-Pueyo, M.; Piñuela, L.; García-Ramos, J. C. (2023). "Deep-sea Ordovician lingulide brachiopods and their associated burrows suggest an early colonization of proximal turbidite systems". Scientific Reports. 13 (1). 22753. Bibcode:2023NatSR..1322753P. doi:10.1038/s41598-023-49875-8. PMC 10733332. PMID 38123596.
- ^ Guo, Z.; Flannery-Sutherland, J. T.; Benton, M. J.; Chen, Z.-Q. (2023). "Bayesian analyses indicate bivalves did not drive the downfall of brachiopods following the Permian-Triassic mass extinction". Nature Communications. 14 (1). 5566. Bibcode:2023NatCo..14.5566G. doi:10.1038/s41467-023-41358-8. PMC 10492784. PMID 37689772.
- ^ Liang, Y.; Strotz, L. C.; Topper, T. P.; Holmer, L. E.; Budd, G. E.; Chen, Y.; Fang, R.; Hu, Y.; Zhang, Z. (2023). "Evolutionary contingency in lingulid brachiopods across mass extinctions". Current Biology. 33 (8): 1565–1572.e3. Bibcode:2023CBio...33E1565L. doi:10.1016/j.cub.2023.02.038. PMID 36893760.
- ^ a b c d e Ausich, W. I.; Wilson, M. A. (2023). "Crinoids from the Wooster Shale Member of the Cuyahoga Formation, Carboniferous (Mississippian, Tournaisian) of northeastern Ohio". Journal of Paleontology. 97 (3): 652–674. Bibcode:2023JPal...97..652A. doi:10.1017/jpa.2023.20. S2CID 258514338.
- ^ Courville, E.; Mooi, R.; Poulin, E.; Saucede, T. (2023). "Arbacia ballenensis sp. nov. (Echinoidea, Arbacioida): A new species reveals diversification of the genus in Central America". Zootaxa. 5336 (4): 555–566. doi:10.11646/zootaxa.5336.4.6. PMID 38221076. S2CID 261143439.
- ^ a b c Courville, E.; González, M.; Mourgues, F. A.; Poulin, E.; Saucède, T. (2023). "New species of the genus Arbacia (Echinoidea, Arbaciidae) from the Neogene of Chile". Ameghiniana. 60 (3): 216–235. doi:10.5710/AMGH.01.03.2023.3536. S2CID 257350390.
- ^ Thuy, B.; Piñuela, L.; García-Ramos, J. C. (2023). "A relict Triassic brittle star (Echinodermata, Ophiuroidea) in Lower Jurassic strata of Asturias, north-west Spain". Swiss Journal of Palaeontology. 142 (1). 10. Bibcode:2023SwJP..142...10T. doi:10.1186/s13358-023-00275-5.
- ^ Zamora, S.; Wright, D. F.; Nohejlová, M. (2023). "Phylogenetic position of Bohemiacinctus gen. nov. (Echinodermata, Cincta) from the Cambrian of Bohemia: implications for macroevolution and the role of taxon sampling in palaeobiological systematics". Papers in Palaeontology. 9 (1). e1482. Bibcode:2023PPal....9E1482Z. doi:10.1002/spp2.1482. hdl:10261/350731. S2CID 257103950.
- ^ a b Ausich, W. I.; Ciampaglio, C.; Fabian, A. J.; Myers, J. R. (2023). "A Silurian (Homerian) pelmatozoan echinoderm fauna from west-central Ohio, USA". Journal of Paleontology. 97 (5): 1070–1091. Bibcode:2023JPal...97.1070A. doi:10.1017/jpa.2023.74.
- ^ a b Schlüter, N.; Wiese, F.; Díaz-Isa, M.; Püttmann, T.; Walaszczyk, I. (2023). "Santonian (Late Cretaceous) echinoids from the Santander area (northern Cantabria, Spain)". Cretaceous Research. 147. 105477. Bibcode:2023CrRes.14705477S. doi:10.1016/j.cretres.2023.105477. S2CID 256266646.
- ^ a b Gale, A. S.; Ishida, Y.; Jagt, J. W. M.; Thuy, B.; Komatsu, T.; Fujita, T. (2023). "A New Asteroid (Echinodermata, Astropectinidae) and Ophiuroid (Echinodermata, Hemieuryalidae) from the Mid-Cretaceous of Southern Japan". Paleontological Research. 28 (2): 1–14. doi:10.2517/PR220020. S2CID 258852447.
- ^ Saucède, T.; Smith, C.; Olivier, N.; Durlet, C.; Gueriau, P.; Thoury, M.; Fara, E.; Escarguel, G.; Brayard, A. (2023). "A new Early Triassic crinoid from Nevada questions the origin and palaeobiogeographical history of dadocrinids". Acta Palaeontologica Polonica. 68 (1): 155–166. doi:10.4202/app.01042.2022. S2CID 257122690.
- ^ Zamora, S.; Gutiérrez-Marco, J. C. (2023). "Filling the Silurian gap of solutan echinoderms with the description of new species of Dehmicystis from Spain". Acta Palaeontologica Polonica. 68 (2): 185–192. doi:10.4202/app.01054.2023. hdl:10261/356026. S2CID 258598979.
- ^ a b c d e Gale, A. S. (2023). "Microcrinoids (Roveacrinidae) from the middle–upper Cenomanian Grey Chalk Subgroup, Dover (Kent, United Kingdom): biostratigraphy and re-evaluation of cup structure in roveacrinids". Acta Geologica Polonica. 73 (4): 685–705. doi:10.24425/agp.2022.143598.
- ^ a b Müller, P.; Hahn, G. (2023). "Erstnachweis von Devonaster Schuchert, 1914 im deutschen Unter-Devon (Asteroidea, Echinodermata)". Mainzer geowissenschaftliche Mitteilungen. 51: 135–152. doi:10.23689/fidgeo-5825.
- ^ a b c Ceccolini, F.; Cianferoni, F. (2023). "New Replacement Names in Fossil Echinoderms (Echinodermata)". Paleontological Research. 27 (4): 379–382. doi:10.2517/PR210029. S2CID 257430558.
- ^ Sałamatin, R.; Kaczmarek, A. (2022). "Astroblastocystis nom. nov. – a new replacement name for Blastocystis Jaekel, 1918 (Echinodermata, Parablastoidea)". Annals of Parasitology. 68 (1): 195–196. doi:10.17420/ap6801.425. PMID 35503892.
- ^ Park, H.; Lee, D.-C. (2023). "Goryeocrinus pentagrammos n. gen. n. sp. (Rhodocrinitidae; Diplobathrida), the first record of camerate crinoid from the Middle Ordovician (Darriwilian) of South Korea (East Gondwana)". Journal of Paleontology. 97 (2): 386–394. Bibcode:2023JPal...97..386P. doi:10.1017/jpa.2022.100. S2CID 256144911.
- ^ Stiller, F. (2023). "A new holocrinid sea lily from the Anisian (Middle Triassic) of Qingyan, south-western China, and the morphological variability of the distal nodal facets in the genus Holocrinus". PalZ. 98: 41–66. doi:10.1007/s12542-023-00661-x. S2CID 260106573.
- ^ Reddy, C.; Thuy, B.; Reid, M.; Gess, R. (2023). "Earliest known ophiuroids from high palaeolatitude, southern Gondwana, recovered from the Pragian to earliest Emsian Baviaanskloof Formation (Table Mountain Group, Cape Supergroup) South Africa". PLOS ONE. 18 (10). e0292636. Bibcode:2023PLoSO..1892636R. doi:10.1371/journal.pone.0292636. PMC 10599496. PMID 37878550.
- ^ Dupichaud, C.; Lefebvre, B.; Milne, C. H.; Mooi, R.; Nohejlová, M.; Roch, R.; Saleh, F.; Zamora, S. (2023). "Solutan echinoderms from the Fezouata Shale Lagerstätte (Lower Ordovician, Morocco): diversity, exceptional preservation, and palaeoecological implications". Frontiers in Ecology and Evolution. 11. 1290063. doi:10.3389/fevo.2023.1290063.
- ^ Kalyakin, E. A.; Barsukov, L. S. (2023). "A new species of Nucleolites (Echinoidea, Cassiduloida) from the Lower Albian of the Russian Plate". Paleontological Journal. 57 (6): 629–636. Bibcode:2023PalJ...57..629K. doi:10.1134/S0031030123060035. S2CID 265500708.
- ^ Thuy, B.; Numberger-Thuy, L. D. (2023). "The Northernmost Occurrence of the Tropical-Subtropical Brittle Star Ophiocoma (Echinodermata, Ophiuroidea) from a Late Cretaceous Rocky Shore in Southern Sweden". Taxonomy. 3 (3): 346–355. doi:10.3390/taxonomy3030020.
- ^ Sweeney, A.; Sumrall, C. D. (2023). "Pleurocystites? scylla, a new species of pleurocystitid rhombiferan, and comments on early echinoderm teratologies". Journal of Paleontology. 97 (3): 631–638. Bibcode:2023JPal...97..631S. doi:10.1017/jpa.2023.17. S2CID 258759477.
- ^ a b Gale, A. S. (2022). "A new "slime star" (Echinodermata, Asteroidea, Velatida) from the Upper Cretaceous Chalk of the United Kingdom". Proceedings of the Geologists' Association. 134: 107–114. doi:10.1016/j.pgeola.2022.12.001. S2CID 254921388.
- ^ Müller, P.; Ausich, W. I. (2023). "A new Periechocrinidae (Crinoidea, Camerata) from the Seifen Formation of the Westerwald (Lower Devonian, Rhenish Massiv, Germany)". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 309 (2): 141–151. doi:10.1127/njgpa/2023/1155. S2CID 261603939.
- ^ Poatskievick Pierezan, B.; Gale, A. S.; Fauth, G. (2023). "A new microcrinoid (Roveacrinidae) from the Aptian–Albian of the Sergipe-Alagoas Basin, northeastern Brazil". Cretaceous Research. 145. 105482. Bibcode:2023CrRes.14505482P. doi:10.1016/j.cretres.2023.105482. S2CID 256442721.
- ^ Ishida, Y.; Tagiri, M.; Kato, T.; Tsunoda, S.; Nakajima, Y.; Thuy, B.; Numberger-Thuy, L. D.; Fujita, T. (2023). "The New Brittle-Star Species Stegophiura takaisoensis (Echinodermata, Ophiuroidea) from the Pliocene of Ibaraki Prefecture, Central Japan". Paleontological Research. 28 (1): 82–96. doi:10.2517/PR220028. S2CID 257960759.
- ^ Salamon, M. A.; Benyoucef, M.; Benzaggah, M.; Brachaniec, T.; Hoşgör, İ̇.; Jain, S.; Płachno, B. J.; Rahmonov, O. (2023). "Unlocking the secrets of the Early Jurassic of North Africa: first record of pseudoplanktonic crinoid Seirocrinus (Crinoidea, Pentacrinidae) from Morocco and Algeria". Historical Biology: An International Journal of Paleobiology. 36 (10): 2174–2186. doi:10.1080/08912963.2023.2243471. S2CID 261087307.
- ^ a b Ishida, Y.; Trinh, H. T.; Thuy, B.; Numberger-Thuy, L. D.; Komatsu, T.; Doan, H. D.; Nguyen, M. T.; Shigeta, Y.; Fujita, T. (2022). "A New Genus and Species of Brittle Star (Ophiuroidea: Ophioleucida) from the Upper Triassic (Carnian) of Northern Vietnam". Paleontological Research. 27 (2): 147–159. doi:10.2517/PR210014. S2CID 253354589.
- ^ Fau, M.; Villier, L. (2023). "Mesozoic stem-group zoroasterid sea stars imply a delayed radiation of the crown group and adaptation to the deep seas". Journal of Systematic Palaeontology. 21 (1). 2243268. Bibcode:2023JSPal..2143268F. doi:10.1080/14772019.2023.2243268. S2CID 262177250.
- ^ Cole, S. R.; Wright, D. F.; Thompson, J. R. (2023). "Calcite-aragonite seas as a driver of echinoderm evolution? Experimental insight and deep-time decoupling". Geology. 51 (12): 1091–1095. Bibcode:2023Geo....51.1091C. doi:10.1130/G51444.1.
- ^ Desatnik, R.; Patterson, Z. J.; Gorzelak, P.; Zamora, S.; LeDuc, P.; Majidi, C. (2023). "Soft robotics informs how an early echinoderm moved". Proceedings of the National Academy of Sciences of the United States of America. 120 (46): e2306580120. Bibcode:2023PNAS..12006580D. doi:10.1073/pnas.2306580120. PMC 10655572. PMID 37931097.
- ^ Álvarez-Armada, N.; Bauer, J. E.; Waters, J. A.; Rahman, I. A. (2023). "The oldest evidence of brooding in a Devonian blastoid reveals the evolution of new reproductive strategies in early echinoderms". Papers in Palaeontology. 9 (3). e1493. Bibcode:2023PPal....9E1493A. doi:10.1002/spp2.1493. hdl:2027.42/176881. S2CID 258810832.
- ^ Guensburg, T. E.; Mooi, R.; Mongiardino Koch, N. (2023). "Crinoid calyx origin from stem radial echinoderms". Journal of Paleontology. 97 (5): 1092–1115. Bibcode:2023JPal...97.1092G. doi:10.1017/jpa.2023.14. S2CID 257851061.
- ^ Hernandez Gomez, N. J.; Melendez, L. E.; Lapic, W. A.; Sheffield, S. L.; Lewis, R. D. (2023). "Examining the ontogeny of the Pennsylvanian cladid crinoid Erisocrinus typus Meek and Worthen, 1865". Journal of Paleontology. 97 (4): 906–913. Bibcode:2023JPal...97..906H. doi:10.1017/jpa.2023.41.
- ^ Gorzelak, P.; Kołbuk, D.; Stolarski, J.; Bącal, P.; Januszewicz, B.; Duda, P.; Środek, D.; Brachaniec, T.; Salamon, M. A. (2023). "A Devonian crinoid with a diamond microlattice". Proceedings of the Royal Society B: Biological Sciences. 290 (1995). 20230092. doi:10.1098/rspb.2023.0092. PMC 10050926. PMID 36987636.
- ^ Salamon, M. A.; Benyoucef, M.; Paszcza, K.; Mekki, F.; Bouchemla, I.; Płachno, B. J. (2023). "The unexpected occurrence of enigmatic 'percevalicrinids' (Echinodermata, Crinoidea) in the Lower Jurassic strata of North Africa — Implications for their stratigraphic and palaeogeographic distribution and discussion on their belonging to the subfamily Balanocrininae". Journal of Palaeogeography. 12 (3): 434–447. Bibcode:2023JPalG..12..434S. doi:10.1016/j.jop.2023.05.002. S2CID 258884395.
- ^ Kolata, D. R.; Frank, T.; Kaplan, A.; Guensburg, T. E. (2023). "New specimens of Cyclocystoides scammaphoris (Echinodermata) from the Upper Ordovician rocks of the American midcontinent with implications for cyclocystoid functional morphology". Journal of Paleontology. 97 (3): 639–651. Bibcode:2023JPal...97..639K. doi:10.1017/jpa.2023.33. S2CID 259449689.
- ^ Wiese, F.; Schlüter, N.; Zirkel, J.; Herrle, J. O.; Friedrich, O. (2023). "A 104-Ma record of deep-sea Atelostomata (Holasterioda, Spatangoida, irregular echinoids) – a story of persistence, food availability and a big bang". PLOS ONE. 18 (8). e0288046. Bibcode:2023PLoSO..1888046W. doi:10.1371/journal.pone.0288046. PMC 10411753. PMID 37556403.
- ^ Sour-Tovar, F.; Quiroz-Barroso, S. A.; Martín-Medrano, L. (2023). "Middle-Upper Permian stenuroid (Asterozoa, Stenuroidea) from Las Delicias Formation, Coahuila, Mexico, relict youngest record for the group". Journal of South American Earth Sciences. 130. 104584. Bibcode:2023JSAES.13004584S. doi:10.1016/j.jsames.2023.104584. S2CID 262028396.
- ^ Thuy, B.; Knox, L.; Numberger-Thuy, L. D.; Smith, N. S.; Sumrall, C. D. (2023). "Ancient deep ocean as a harbor of biotic innovation revealed by Carboniferous ophiuroid microfossils". Geology. doi:10.1130/G50596.1.
- ^ a b c d e Maletz, J. (2023). The Lower Ordovician (Tremadocian to Floian) graptolite fauna of Hunneberg, Västergötland, Sweden. Fossils and Strata Series. Vol. 69. pp. 1–140. doi:10.18261/9788215070872-2023. ISBN 978-8-215-07086-5. S2CID 265338481.
- ^ a b c Maletz, J. (2023). "Retiolitid graptolites from the collection of Hermann Jaeger III. Paraplectograptus, Gothograptus and their relatives". PalZ. 97 (2): 323–352. Bibcode:2023PalZ...97..323M. doi:10.1007/s12542-022-00646-2. S2CID 256880454.
- ^ Briggs, D. E. G.; Mongiardino Koch, N. (2023). "A Silurian pseudocolonial pterobranch". Current Biology. 33 (23): 5225–5232.e3. Bibcode:2023CBio...33E5225B. doi:10.1016/j.cub.2023.10.024. PMID 37935193. S2CID 265037290.
- ^ Nanglu, K.; Waskom, M. E.; Richards, J. C.; Ortega-Hernández, J. (2023). "Rhabdopleurid epibionts from the Ordovician Fezouata Shale biota and the longevity of cross-phylum interactions". Communications Biology. 6 (1). 1002. doi:10.1038/s42003-023-05377-x. PMC 10567727. PMID 37821659.
- ^ a b Maletz, J.; Gutiérrez-Marco, J. C. (2024). "The purported record of an epibiontic rhabdopleurid in the Early Ordovician Fezouata biota of Morocco, with a discussion about benthic pterobranchs (Hemichordata) in the Lagerstätte". Geobios. doi:10.1016/j.geobios.2024.09.001.
- ^ Lopez, F. E.; Kaufmann, C.; Drovandi, J. M.; Conde, O. A.; Braeckman, A. R.; Arnol, J. A.; Estrada, L.; Pedernera, F.; Abarca, U. (2023). "First record of Pridolian graptolites from South America: biostratigraphic and paleogeographic remarks". Gondwana Research. 119: 246–261. Bibcode:2023GondR.119..246L. doi:10.1016/j.gr.2023.03.026. S2CID 258046676.
- ^ Rueda, E. K.; Albanesi, G. L. (2023). "Fryxellodontus inornatus (Conodonta) and associated conodonts from the Furongian (upper Cambrian) of the Cordillera Oriental, Argentina". Palaeoworld. 33 (4): 839–856. doi:10.1016/j.palwor.2023.06.003. S2CID 259641637.
- ^ Lu, J.-F. (2023). "Conodonts across the Lochkovian/Pragian boundary in central Guangxi, South China". Palaeoworld. 33 (5): 1268–1280. doi:10.1016/j.palwor.2023.11.002. S2CID 265136458.
- ^ a b c d e Albanesi, G. L.; Rubén Monaldi, C.; Barnes, C. R.; Zeballo, F. J.; Ortega, G. (2023). "An endemic conodont fauna of Darriwilian (Middle Ordovician) age from the Santa Gertrudis Formation, southwestern Gondwanan margin and its paleobiogeographic relationships". Marine Micropaleontology. 181. 102241. Bibcode:2023MarMP.181j2241A. doi:10.1016/j.marmicro.2023.102241. S2CID 257863952.
- ^ Chen, A.; Zhang, Y.; Golding, M. L.; Wu, H.; Liu, J. (2023). "Upper Changhsingian to lower Anisian conodont biostratigraphy of the Datuguan section, Nanpanjiang Basin, South China". Palaeogeography, Palaeoclimatology, Palaeoecology. 616. 111470. Bibcode:2023PPP...61611470C. doi:10.1016/j.palaeo.2023.111470.
- ^ a b c Izokh, N. G. (2023). "New Givetian conodonts of the genus Icriodus from the Kolyvan-Tom folded area (south of west Siberia)". Paleontological Journal. 57 (5): 553–559. Bibcode:2023PalJ...57..553I. doi:10.1134/S0031030123050027. S2CID 262543076.
- ^ a b c d e f g h i Yuan, Z.; Sun, Y. (2023). "Upper Devonian conodont fauna from Western Hubei, China and its significance". Historical Biology: An International Journal of Paleobiology. 36 (5): 954–980. doi:10.1080/08912963.2023.2199763. S2CID 258394745.
- ^ a b Lyu, S.; Henderson, C. M.; Chen, Z.-Q.; Tong, J.; Zhao, L.; Han, C.; Wu, S. (2023). "High-resolution conodont unitary association zonations (UAZs) across the Induan-Olenekian boundary (Lower Triassic): A global correlation". Palaeogeography, Palaeoclimatology, Palaeoecology. 627. 111721. Bibcode:2023PPP...62711721L. doi:10.1016/j.palaeo.2023.111721.
- ^ Wu, S.; Golding, M. L.; Zhao, H.; Han, C.; Zhang, X.; Chen, Z.-Q.; Lyu, Z. (2023). "Lower to Middle Triassic conodont biostratigraphy and carbonate carbon isotope chemostratigraphy of the Zuodeng area, Guangxi, South China, and its relevance for stratigraphic correlation from the Induan to the Anisian". Palaeogeography, Palaeoclimatology, Palaeoecology. 636. 111965. doi:10.1016/j.palaeo.2023.111965.
- ^ Leu, M. (2024). "Establishment and Priority of Neospathodus bevelledi over Neospathodus yangtzeensis: Clarification and Recommendations for Future Nomenclature and Stratigraphic Naming". zenodo.org. doi:10.5281/zenodo.10794343. Retrieved 2024-07-31.
- ^ a b Lu, J.-F.; Guo, W.; Wang, Y.; Xu, H.-H. (2023). "The first discovery of Lochkovian (Lower Devonian) conodonts in central Guangxi, South China and its geological implications". Journal of Paleontology. 97 (2): 421–433. Bibcode:2023JPal...97..421L. doi:10.1017/jpa.2023.2. S2CID 256928406.
- ^ Pei, F.; Ba, Y. (2023). "Late Cambrian and Early Ordovician conodonts from Hupinao of Cixian County, Hebei Province". Acta Micropalaeontologica Sinica. 40 (3): 206–215. doi:10.16087/j.cnki.1000-0674.20230816.001.
- ^ Zhen, Y. Y. (2023). "Revision of the Ordovician conodont species Fahraeusodus adentatus and the new genus Pohlerodus". Alcheringa: An Australasian Journal of Palaeontology. 47 (1): 11–23. Bibcode:2023Alch...47...11Z. doi:10.1080/03115518.2023.2172210. S2CID 257189945.
- ^ Suttner, T. J.; Uugantsetseg, B.; Ariunchimeg, Y.; Manchuk, N.; Kido, E.; Erdenejargal, C.; Buyantegsh, B.; Enkhbaatar, B.; Zorig, E. (2023). "Case study on Hangenberg Crisis equivalent deposits and associated conodont faunas including Siphonodella progenitors from late Devonian island arc settings (Indert Formation, Shine Jinst region, southern Mongolia)". Newsletters on Stratigraphy. 57: 65–88. doi:10.1127/nos/2023/0760. S2CID 259197704.
- ^ Huang, Y.-Z.; Guo, W.; Lin, W.; Zhaxi, P.-C.; Yao, L.; Hu, K.-Y.; Wang, Q.-L.; Qi, Y.-P. (2023). "First record of the late Famennian conodonts in Qamdo, Tibet and their biostratigraphic implications". Palaeoworld. 33 (3): 624–635. doi:10.1016/j.palwor.2023.09.001. S2CID 261687562.
- ^ Zhen, Y. Y.; Zhang, Y.-D.; Chen, Z.-Y.; Wang, L.-W. (2023). "Origin and evolution of the Early Ordovician conodont genus Prioniodus Pander, 1856 — New evidence from South China". Marine Micropaleontology. 183. 102269. doi:10.1016/j.marmicro.2023.102269. S2CID 260221500.
- ^ Plotitsyn, A. N.; Zhuravlev, A. V. (2023). "Model of phylomorphogeny of P1 elements of the Tournaisian (Mississippian) siphonodellids". Marine Micropaleontology. 184. 102294. Bibcode:2023MarMP.18402294P. doi:10.1016/j.marmicro.2023.102294. S2CID 262223110.
- ^ Girard, C.; Charruault, A.-L.; Dufour, A.-B.; Renaud, S. (2023). "Conodont size in time and space: Beyond the temperature-size rule". Marine Micropaleontology. 184. 102291. Bibcode:2023MarMP.18402291G. doi:10.1016/j.marmicro.2023.102291. S2CID 261636630.
- ^ Wu, K.; Zou, Y.; Li, H.; Wan, S.; Yang, L.; Cui, Y.; Li, J.; Zhao, B.; Cheng, L. (2023). "A unique early Triassic (Spathian) conodont community from the Nanzhang-Yuan'an Fauna, Hubei Province, South China". Geological Journal. 58 (10): 3879–3898. Bibcode:2023GeolJ..58.3879W. doi:10.1002/gj.4815. S2CID 259425064.
- ^ Kelz, V.; Guenser, P.; Rigo, M.; Jarochowska, E. (2023). "Growth allometry and dental topography in Upper Triassic conodonts support trophic differentiation and molar-like element function". Paleobiology. 49 (4): 665–683. Bibcode:2023Pbio...49..665K. doi:10.1017/pab.2023.8. hdl:1874/433236. S2CID 235414143.
- ^ Zeng, W.; Jiang, H.; Chen, Y.; Ogg, J.; Zhang, M.; Dong, H. (2023). "Upper Norian conodonts from the Baoshan block, western Yunnan, southwestern China, and implications for conodont turnover". PeerJ. 11. e14517. doi:10.7717/peerj.14517. PMC 9854380. PMID 36684668.
- ^ Du, Y.; Onoue, T.; Tomimatsu, Y.; Wu, Q.; Rigo, M. (2023). "Lower Jurassic conodonts from the Inuyama area of Japan: implications for conodont extinction". Frontiers in Ecology and Evolution. 11. 1135789. doi:10.3389/fevo.2023.1135789. hdl:11577/3479836.
- ^ Hart, L. J.; Gee, B. M.; Smith, P. M.; McCurry, M. R. (2023). "A new chigutisaurid (Brachyopoidea, Temnospondyli) with soft tissue preservation from the Triassic Sydney Basin, New South Wales, Australia". Journal of Vertebrate Paleontology. 42 (6). e2232829. doi:10.1080/02724634.2023.2232829. S2CID 260642293.
- ^ Chakravorti, Sanjukta; Sengupta, Dhurjati Prasad (2023-03-06). "The first record of chigutisaurid amphibian from the Late Triassic Tiki Formation and the probable Carnian pluvial episode in central India". PeerJ. 11: e14865. doi:10.7717/peerj.14865. ISSN 2167-8359. PMC 9997194. PMID 36908823.
- ^ Kligman, Ben T.; Gee, Bryan M.; Marsh, Adam D.; Nesbitt, Sterling J.; Smith, Matthew E.; Parker, William G.; Stocker, Michelle R. (2023-01-25). "Triassic stem caecilian supports dissorophoid origin of living amphibians". Nature. 614 (7946): 102–107. Bibcode:2023Natur.614..102K. doi:10.1038/s41586-022-05646-5. ISSN 1476-4687. PMC 9892002. PMID 36697827.
- ^ Zhang, J.; Dong, L.; Du, B.; Li, A.; Lei, X.; Zhang, M.; Wang, S.; Ma, G.; Hui, J. (2023). "First fossil evidence for a new frog from the Early Cretaceous of the Jiuquan Basin, Gansu Province, north-western China". Journal of Systematic Palaeontology. 21 (1). 2183146. Bibcode:2023JSPal..2183146Z. doi:10.1080/14772019.2023.2183146. S2CID 257647888.
- ^ Lemierre, A.; Bailon, S.; Folie, A.; Laurin, M. (2023). "A new pipid from the Cretaceous of Africa (In Becetèn, Niger) and early evolution of the Pipidae". Journal of Systematic Palaeontology. 21 (1). 2266428. Bibcode:2023JSPal..2166428L. doi:10.1080/14772019.2023.2266428. S2CID 265476228.
- ^ Skutschas, P.; Kolchanov, V.; Anpilogova, E.; Parakhin, I.; Averianov, A.; Jones, M. (2023). "The last of them? A new relic karaurid stem salamander from the Lower Cretaceous of Western Siberia, Russia". Biological Communications. 68 (4): 219–226. doi:10.21638/spbu03.2023.402.
- ^ Turazzini, G. F.; Gómez, R. O. (2023). "A new old Budgett frog: an articulated skeleton of an Early Pliocene Lepidobatrachus (Anura, Ceratophryidae) from western Argentina". Journal of Vertebrate Paleontology. 42 (5). e2207092. doi:10.1080/02724634.2023.2207092. S2CID 259335339.
- ^ Santos, R. O.; Carvalho, A. B.; Zaher, H. (2023). "A new fossil frog (Lissamphibia: Anura) from the Late Cretaceous of Brazil and the early evolution of neobatrachians". Zoological Journal of the Linnean Society. 202. doi:10.1093/zoolinnean/zlad167.
- ^ Werneburg, R.; Schneider, J. W.; Štamberg, S.; Legler, B.; Schoch, R. R. (2023). "A new amphibamiform (Temnospondyli: Branchiosauridae) from the lower Permian of the Czech Boskovice Basin". Journal of Vertebrate Paleontology. 42 (6). e2231994. doi:10.1080/02724634.2023.2231994. S2CID 260397578.
- ^ Gee, B. M.; Beightol, C. V.; Sidor, C. A. (2023). "A new lapillopsid from Antarctica and a reappraisal of the phylogenetic relationships of early diverging stereospondyls". Journal of Vertebrate Paleontology. 42 (6). e2216260. doi:10.1080/02724634.2023.2216260. S2CID 259688079.
- ^ Schoch, R. R.; Werneburg, R. (2023). "Adult branchiosaurid temnospondyls: the life cycle of Xerodromeus gracilis". Papers in Palaeontology. 9 (4). e1513. Bibcode:2023PPal....9E1513S. doi:10.1002/spp2.1513. S2CID 259871912.
- ^ Porro, L. B.; Rayfield, E. J.; Clack, J. A. (2023). "Computed tomography and three-dimensional reconstruction of the skull of the stem tetrapod Crassigyrinus scoticus Watson, 1929". Journal of Vertebrate Paleontology. 42 (4). e2183134. doi:10.1080/02724634.2023.2183134. S2CID 258475146.
- ^ Pardo, J. D. (2023). "New information on the neurocranium of Archeria crassidisca and the relationships of the Embolomeri". Zoological Journal of the Linnean Society. 201 (3). doi:10.1093/zoolinnean/zlad156.
- ^ Witzmann, F.; Fröbisch, N. (2023). "Morphology and ontogeny of carpus and tarsus in stereospondylomorph temnospondyls". PeerJ. 11. e16182. doi:10.7717/peerj.16182. PMC 10613440. PMID 37904842.
- ^ Werneburg, R.; Stapf, A.; Stapf, H.; Will, V.; Göltz, G.; Raisch, M. (2023). "Earliest ontogeny of Sclerocephalus (Stereospondylomorpha) completed". Semana. Naturwissenschaftliche Veröffentlichungen des Naturhistorischen Museums Schloss Bertholdsburg Schleusingen. 38: 71–93.
- ^ Groenewald, D. P.; Krüger, A.; Day, M. O.; Penn-Clarke, C. R.; Hancox, P. J.; Rubidge, B. S. (2023). "Unique trackway on Permian Karoo shoreline provides evidence of temnospondyl locomotory behaviour". PLOS ONE. 18 (3). e0282354. Bibcode:2023PLoSO..1882354G. doi:10.1371/journal.pone.0282354. PMC 10057796. PMID 36989249.
- ^ Morkovin, B. I. (2023). "Incomplete Large Lower Jaw of Vladlenosaurus alexeyevi from the Lower Triassic of the Luza River (Komi Republic, Russia) and Growth Features in Temnospondyl Amphibians". Paleontological Journal. 56 (11): 1482–1490. doi:10.1134/S0031030122110107. S2CID 256618570.
- ^ Schoch, R. R.; Milner, A.; Witzmann, F.; Mujal, E. (2023). "A revision of Mastodonsaurus from the Anisian of Germany, and the evolutionary history of mastodonsaurid temnospondyls". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 309 (2): 123–140. doi:10.1127/njgpa/2023/1154. S2CID 261594499.
- ^ Teschner, E. M.; Garbay, L.; Janecki, P.; Konietzko-Meier, D. (2023). "Palaeohistology helps reveal taxonomic variability in exceptionally large temnospondyl humeri from the Upper Triassic of Krasiejów, SW Poland". Acta Palaeontologica Polonica. 68 (1): 63–74. doi:10.4202/app.01027.2022. S2CID 256865819.
- ^ Rinehart, L. F.; Lucas, S. G. (2023). "Lateral skull angle: a new sexual dimorphism signal in temnospondyl amphibians". New Mexico Museum of Natural History and Science Bulletin. 94: 559–583.
- ^ Syromyatnikova, E. V. (2023). "Review of Records of Mioproteus (Caudata: Proteidae) from Southeastern Europe". Paleontological Journal. 56 (11): 1428–1436. doi:10.1134/S0031030122110193. S2CID 256618577.
- ^ Skutschas, P. P.; Kolchanov, V. V.; Averianov, A. O.; Schellhorn, R.; Kolosov, P. N.; Jones, M. E. H.; Martin, T. (2023). "The northernmost occurrence of non-karaurid salamanders (Lissamphibia, Caudata) in the Mesozoic". Cretaceous Research. 152. 105686. Bibcode:2023CrRes.15205686S. doi:10.1016/j.cretres.2023.105686. S2CID 261265046.
- ^ Bourque, J. R.; Stanley, E. L.; Hulbert, R. C. (2023). "A Late Miocene occurrence of the extinct salamander Batrachosauroides (Caudata, Batrachosauroididae) and other new caudate fossils from Florida and Georgia, USA". Bulletin of the Florida Museum of Natural History. 60 (4): 235–255. doi:10.58782/flmnh.tzqg4599.
- ^ Pérez-Ben, C. M.; Lires, A. I.; Gómez, R. O. (2023). "Frog limbs in deep time: is jumping locomotion at the roots of the anuran Bauplan?". Paleobiology. 50: 96–107. doi:10.1017/pab.2023.23. S2CID 261993616.
- ^ Roček, Z.; Dong, L.; Wang, Y. (2023). "The Early Cretaceous frog Genibatrachus from China: Osteology, development, and palaeogeographic relations". Palaeobiodiversity and Palaeoenvironments. 103 (4): 799–825. Bibcode:2023PdPe..103..799R. doi:10.1007/s12549-023-00579-x. S2CID 258389641.
- ^ Skutschas, P. P.; Kolchanov, V. V.; Bolotsky, I. Y.; Kuzmin, I. T.; Grigoriev, D. V.; Bapinaev, R. A.; Vitenko, D. D.; Mazur, E. V.; Parakhin, I. A.; Gvozdkova, V. A.; Lubchenkov, D. A.; Bogoy, A. P.; Bolotsky, Y. L. (2023). "The First Findings of Frogs (Anura) from the Upper Cretaceous in Russia". Doklady Earth Sciences. 510 (2): 465–467. Bibcode:2023DokES.510..465S. doi:10.1134/S1028334X2360041X. S2CID 259157273.
- ^ Báez, A. M.; Turazzini, G. F. (2023). "New data on the osteology and development of Avitabatrachus uliana (Anura, Xenoanura), a pipimorph from the Candeleros Formation, mid Cretaceous of northwestern Patagonia, Argentina". Ameghiniana. 61 (1): 25–33. doi:10.5710/AMGH.08.09.2023.3567. S2CID 261788850.
- ^ Vallejo-Pareja, M. C.; Stanley, E. L.; Bloch, J. I.; Blackburn, D. C. (2023). "Fossil frogs (Eleutherodactylidae: Eleutherodactylus) from Florida suggest overwater dispersal from the Caribbean by the Late Oligocene". Zoological Journal of the Linnean Society. 201 (2): 431–446. doi:10.1093/zoolinnean/zlad130.
- ^ Georgalis, G. L.; Prendini, E.; Roček, Z. (2023). "New information on the Eocene frog Thaumastosaurus (Anura, Pyxicephalidae) from the Phosphorites du Quercy, France". Zoological Journal of the Linnean Society. 199 (3): 744–770. doi:10.1093/zoolinnean/zlad047.
- ^ Lemierre, A.; Gendry, D.; Poirier, M.-M.; Gillet, V.; Vullo, R. (2023). "The oldest articulated ranid from Europe: a Pelophylax specimen from the lowest Oligocene of Chartres-de-Bretagne (N.W. France)". Journal of Vertebrate Paleontology. 42 (4). e2191663. doi:10.1080/02724634.2023.2191663. S2CID 258255431.
- ^ Jansen, O.; Gómez, R. O.; Fouquet, A.; Marivaux, L.; Salas-Gismondi, R.; Antoine, P.-O. (2023). "First Eocene–Miocene anuran fossils from Peruvian Amazonia: insights into neotropical frog evolution and diversity". Papers in Palaeontology. 9 (6). e1542. Bibcode:2023PPal....9E1542J. doi:10.1002/spp2.1542. S2CID 266450631.
- ^ Klembara, J.; Werneburg, R.; Mikudíková, M.; Šurka, J.; Štamberg, S. (2023). "The oldest records of the stem amniote Discosauriscus (Seymouriamorpha, Discosauriscidae) from the European Carboniferous-Permian boundary". Bulletin of Geosciences. 98 (3): 233–246. doi:10.3140/bull.geosci.1882.
- ^ Bazzana-Adams, K. D.; Evans, D. C.; Bevitt, J. J.; Reisz, R. R. (2023). "Neurosensory anatomy and function in Seymouria". Journal of Morphology. 284 (5). e21577. doi:10.1002/jmor.21577. PMID 36921082. S2CID 257564333.
- ^ Barták, P.; Ivanov, M. (2023). "The exceptionally well-preserved Sauropleura scalaris (Nectridea: Urocordylidae) from the late Carboniferous of the Czech Republic: new information on ontogeny, lateral line and tail". Zoological Journal of the Linnean Society. 199 (2): 392–416. doi:10.1093/zoolinnean/zlad039.
- ^ Bulanov, V. V. (2023). "The discovery of diadectomorph tetrapods in the Lower Permian of Eastern Europe". Paleontological Journal. 57 (2): 206–216. Bibcode:2023PalJ...57..206B. doi:10.1134/S0031030123020065. S2CID 258640526.
- ^ Calábková, G.; Madzia, D.; Nosek, V.; Ivanov, M. (2023). "Tracking 'transitional' diadectomorphs in the earliest Permian of equatorial Pangea". PeerJ. 11. e16603. doi:10.7717/peerj.16603. PMC 10710172. PMID 38077424.
- ^ Mueller, B. D.; Huttenlocker, A. K.; Small, B. J.; Pinto, J. L.; Dean-Wallace, K.; Chatterjee, S. (2023). "A new kannemeyeriiform dicynodont (Synapsida) from a Late Triassic vertebrate assemblage in west Texas, U.S.A." Journal of Vertebrate Paleontology. 43 (2). e2255236. doi:10.1080/02724634.2023.2255236.
- ^ a b Sidor, C. A. (2023). "New and historical specimens of burnetiamorph therapsids, with comments on ontogeny, biogeography, and bizarre structures". Palaeontologia Africana. 56: 16–35. hdl:10539/35696.
- ^ Kammerer, C. F. (2023). "Revision of the Scylacosauridae (Therapsida: Therocephalia)". Palaeontologia Africana. 56: 51–87. hdl:10539/35700.
- ^ Kammerer, Christian; Viglietti, Pia; Butler, Elize; Botha, Jennifer (22 May 2023). "Rapid turnover of top predators in African terrestrial faunas around the Permian-Triassic mass extinction". Current Biology. 33 (11): 2283–2290.e3. Bibcode:2023CBio...33E2283K. doi:10.1016/j.cub.2023.04.007. PMID 37220743. S2CID 258835757.
- ^ Shi, Y.; Liu, J. (2023). "The tetrapod fauna of the upper Permian Naobaogou Formation of China: 10. Jimusaria monanensis sp. nov. (Dicynodontia) shows a unique epipterygoid". PeerJ. 11. e15783. doi:10.7717/peerj.15783. PMC 10399559. PMID 37547715.
- ^ a b Suchkova, Yu. A.; Golubev, V. K.; Shumov, I. S. (2022). "New Primitive Therocephalians from the Permian of Eastern Europe". Paleontological Journal. 56 (11): 1419–1427. Bibcode:2022PalJ...56.1419S. doi:10.1134/S0031030122110181. S2CID 256618344.
- ^ Mann, A.; Henrici, A. C.; Sues, H.-D.; Pierce, S. E. (2023). "A new Carboniferous edaphosaurid and the origin of herbivory in mammal forerunners". Scientific Reports. 13 (1). 4459. Bibcode:2023NatSR..13.4459M. doi:10.1038/s41598-023-30626-8. PMC 10076360. PMID 37019927.
- ^ Liu, J.; Abdala, F. (2023). "Late Permian terrestrial faunal connections invigorated: the first whaitsioid therocephalian from China". Palaeontologia Africana. 56: 16–35. hdl:10539/35706.
- ^ Day, M. O.; Kammerer, C. F. (2023). "Reappraisal of supposed 'dinocephalian' specimens expands burnetiamorph diversity in the Guadalupian Tapinocephalus Assemblage Zone of South Africa". Palaeontologia Africana. 56: 36–50. hdl:10539/35698.
- ^ Schmitt, Maurício Rodrigo; Martinelli, Agustín Guillermo; Kaiuca, João Felipe Leal; Schultz, Cesar Leandro; Soares, Marina Bento (2023-05-29). "Old fossil findings in the Upper Triassic rocks of southern Brazil improve diversity of traversodontid cynodonts ( Therapsida , Cynodontia )". The Anatomical Record. 307 (4): 1474–1514. doi:10.1002/ar.25244. ISSN 1932-8486. PMID 37246488. S2CID 258960737.
- ^ Szczygielski, T.; Sulej, T. (2023). "Woznikella triradiata n. gen., n. sp. – a new kannemeyeriiform dicynodont from the Late Triassic of northern Pangea and the global distribution of Triassic dicynodonts". Comptes Rendus Palevol (in French). 22 (16): 279–406. doi:10.5852/cr-palevol2023v22a16. S2CID 258752843.
- ^ Harano, T.; Asahara, M. (2023). "Revisiting the evolutionary trend toward the mammalian lower jaw in non-mammalian synapsids in a phylogenetic context". PeerJ. 11. e15575. doi:10.7717/peerj.15575. PMC 10289081. PMID 37361048.
- ^ Bishop, P. J.; Pierce, S. E. (2023). "The fossil record of appendicular muscle evolution in Synapsida on the line to mammals: Part I—Forelimb". The Anatomical Record. 307 (5): 1764–1825. doi:10.1002/ar.25312. PMID 37726984. S2CID 262068960.
- ^ Bishop, P. J.; Pierce, S. E. (2023). "The fossil record of appendicular muscle evolution in Synapsida on the line to mammals: Part II—Hindlimb". The Anatomical Record. 307 (5): 1826–1896. doi:10.1002/ar.25310. PMID 37727023.
- ^ Hellert, S. M.; Grossnickle, D. M.; Lloyd, G. T.; Kammerer, C. F.; Angielczyk, K. D. (2023). "Derived faunivores are the forerunners of major synapsid radiations". Nature Ecology & Evolution. 7 (11): 1903–1913. Bibcode:2023NatEE...7.1903H. doi:10.1038/s41559-023-02200-y. PMID 37798433.
- ^ Calábková, G.; Březina, J.; Nosek, V.; Madzia, D. (2023). "Synapsid tracks with skin impressions illuminate the terrestrial tetrapod diversity in the earliest Permian of equatorial Pangea". Scientific Reports. 13 (1). 1130. Bibcode:2023NatSR..13.1130C. doi:10.1038/s41598-023-27939-z. PMC 9860047. PMID 36670191.
- ^ Maho, T.; Bevitt, J. J.; Reisz, R. R. (2023). "New specimens of the early Permian apex predator Varanops brevirostris at Richards Spur, Oklahoma, with histological information about its growth pattern". PeerJ. 11. e14898. doi:10.7717/peerj.14898. PMC 9938655. PMID 36819993.
- ^ Gônet, J.; Bardin, J.; Girondot, M.; Hutchinson, J. R.; Laurin, M. (2023). "Unravelling the postural diversity of mammals: Contribution of humeral cross-sections to palaeobiological inferences". Journal of Mammalian Evolution. 30 (2): 321–337. doi:10.1007/s10914-023-09652-w. S2CID 256788973.
- ^ Bazzana-Adams, K. D.; Evans, D. C.; Reisz, R. R. (2023). "Neurosensory anatomy and function in Dimetrodon, the first terrestrial apex predator". iScience. 26 (4): 106473. Bibcode:2023iSci...26j6473B. doi:10.1016/j.isci.2023.106473. PMC 10122045. PMID 37096050. S2CID 257678720.
- ^ Bishop, P. J.; Norton, L. A.; Jirah, S.; Day, M. O.; Rubidge, B. S.; Pierce, S. E. (2023). "Enigmatic humerus from the mid-Permian of South Africa bridges the anatomical gap between "pelycosaurs" and therapsids". Journal of Vertebrate Paleontology. 42 (3). e2170805. doi:10.1080/02724634.2023.2170805. S2CID 257338054.
- ^ Santos, M. A. C.; Paes Neto, V. D.; Schultz, C. L.; Cisneros, J.; Pierce, S. E.; Pinheiro, F. L. (2023). "Cranial osteology of the Brazilian dinocephalian Pampaphoneus biccai (Anteosauridae: Syodontinae)". Zoological Journal of the Linnean Society. 199 (4): 1034–1058. doi:10.1093/zoolinnean/zlad071.
- ^ Benoit, J.; Norton, L. A.; Jirah, S. (2023). "The maxillary canal of the titanosuchid Jonkeria (Synapsida, Dinocephalia)". The Science of Nature. 110 (4). 27. Bibcode:2023SciNa.110...27B. doi:10.1007/s00114-023-01853-w. PMC 10241669. PMID 37272962.
- ^ Rubidge, B. S.; Day, M. O.; Benoit, J. (2023). "First record of the rare dicynodont Colobodectes from the southern Karoo Basin of South Africa has implications for middle Permian continental biostratigraphy". Journal of African Earth Sciences. 208. 105097. Bibcode:2023JAfES.20805097R. doi:10.1016/j.jafrearsci.2023.105097. S2CID 264502705.
- ^ a b Laaß, M.; Kaestner, A. (2023). "Nasal turbinates of the dicynodont Kawingasaurus fossilis and the possible impact of the fossorial habitat on the evolution of endothermy". Journal of Morphology. 284 (9). e21621. doi:10.1002/jmor.21621. PMID 37585231. S2CID 260360508.
- ^ Macungo, Z.; Araújo, R.; Browning, C.; Smith, R. M. H.; David, R.; Angielczyk, K.; Massingue, A.; Ferreira-Cardoso, S.; Kortje, D. J. P. (2023). "Novel anatomy and paleobiological insights on Cistecephalus microrhinus (Synapsida: Dicynodontia)". In Yuong-Nam Lee (ed.). Windows into sauropsid and synapsid evolution. Essays in honor of Louis L. Jacobs. Dinosaur Science Center Press. pp. 1–65. ISBN 978-89-5708-358-1.
- ^ Sidor, C. A.; Mann, A.; Angielczyk, K. D. (2023). "Gorgonops and Endothiodon (Synapsida: Therapsida) from the Madumabisa Mudstone Formation: evidence of a previously unreported tetrapod biozone in the Mid-Zambezi Basin of southern Zambia". Journal of Vertebrate Paleontology. 43 (1). e2256812. doi:10.1080/02724634.2023.2256812. S2CID 265506958.
- ^ Bendel, E.-M.; Kammerer, C. F.; Smith, R. M. H.; Fröbisch, J. (2023). "The postcranial anatomy of Gorgonops torvus (Synapsida, Gorgonopsia) from the late Permian of South Africa". PeerJ. 11. e15378. doi:10.7717/peerj.15378. PMC 10332358. PMID 37434869.
- ^ Mocke, H. B.; Kammerer, C. F.; Smith, R. M. H.; Marsicano, C. A. (2023). "The first record of late Permian tetrapods from Namibia". Palaeontologia Africana. 56: 133–141. hdl:10539/37140.
- ^ Groenewald, D. P.; Kammerer, C. F. (2023). "Re-identification and updated stratigraphic context of the holotypes of the late Permian tetrapods Dicynodon ingens and Scymnosaurus warreni from KwaZulu-Natal". Palaeontologia Africana. 56: 171–179. hdl:10539/37143.
- ^ Gigliotti, A.; Pusch, L. C.; Kammerer, C. F.; Benoit, J.; Fröbisch, J. (2023). "Craniomandibular anatomy of the akidnognathid therocephalian Olivierosuchus parringtoni from the Early Triassic of South Africa". Palaeontologia Africana. 56: 142–170. hdl:10539/37185.
- ^ Abdala, F.; Hendrickx, C.; Jasinoski, S. C.; Gaetano, L. C.; Liu, J. (2023). "The pre-eminence of the Karoo Basin in the knowledge of the Permo-Jurassic cynodonts: a historical synthesis and taxonomical quantification". Palaeontologia Africana. 56: 213–227. hdl:10539/37136.
- ^ Pusch, L. C.; Kammerer, C. F.; Fernandez, V.; Fröbisch, J. (2023). "Cranial anatomy of Nythosaurus larvatus Owen, 1876, an Early Triassic cynodont preserving a natural endocast". Journal of Vertebrate Paleontology. 42 (3). e2174441. doi:10.1080/02724634.2023.2174441. S2CID 257419409.
- ^ Kulik, Z. T. (2023). "Rare osteohistological evidence of skeletal maturity in the early diverging traversodontid Scalenodon angustifrons, with comments on histological sampling coverage in Cynodontia". Journal of Vertebrate Paleontology. 43 (1). e2249964. doi:10.1080/02724634.2023.2249964. S2CID 265506987.
- ^ Hoffmann, C. A.; Ribeiro, A. M.; de Andrade, M. B. (2023). "On the dentition, tooth replacement, and taxonomic status of Charruodon tetracuspidatus Abdala & Ribeiro, 2000: A bizarre cynodont from the middle upper Triassic of southern Brazil". The Anatomical Record. 307 (4): 1524–1537. doi:10.1002/ar.25349. PMID 37950602.
- ^ Hoffmann, C. A.; de Andrade, M. B.; Martinelli, A. G. (2023). "Anatomy of the holotype of 'Probelesodon' kitchingi revisited, a chiniquodontid cynodont (Synapsida, Probainognathia) from the early Late Triassic of southern Brazil". Journal of Paleontology. 97 (3): 693–710. Bibcode:2023JPal...97..693H. doi:10.1017/jpa.2023.25. S2CID 258871869.
- ^ Kerber, L.; Pretto, F. A.; Müller, R. T. (2023). "New information on the mandibular anatomy of Agudotherium gassenae, a Late Triassic non-mammaliaform probainognathian from Brazil". The Anatomical Record. 307 (4): 1515–1523. doi:10.1002/ar.25317. PMID 37767852. S2CID 263117209.
- ^ Stefanello, M.; Martinelli, A. G.; Müller, R. T.; Dias-da-Silva, S.; Kerber, L. (2023). "A complete skull of a stem mammal from the Late Triassic of Brazil illuminates the early evolution of prozostrodontian cynodonts". Journal of Mammalian Evolution. 30 (2): 299–317. doi:10.1007/s10914-022-09648-y. S2CID 256452176.
- ^ Kerber, L.; Roese-Miron, L.; Bubadué, J. M.; Martinelli, A. G. (2023). "Endocranial anatomy of the early prozostrodonts (Eucynodontia: Probainognathia) and the neurosensory evolution of the mammalian forerunners". The Anatomical Record. 307 (4): 1442–1473. doi:10.1002/ar.25215. PMID 37017195. S2CID 257954339.
- ^ Lund, E. S.; Norton, L. A.; Benoit, J. (2023). "First CT-assisted study of the palate and postcrania of Diarthrognathus broomi (Cynodontia, Probainognathia)". The Anatomical Record. 307 (4): 1538–1558. doi:10.1002/ar.25363. PMID 38131650.
- ^ Lautenschlager, S.; Fagan, M. J.; Luo, Z.-X.; Bird, C. M.; Gill, P.; Rayfield, E. J. (2023). "Functional reorganisation of the cranial skeleton during the cynodont–mammaliaform transition". Communications Biology. 6 (1). 367. doi:10.1038/s42003-023-04742-0. PMC 10097706. PMID 37046052.
- ^ Martin, T.; Schultz, J. A. (2023). "Deciduous dentition, tooth replacement, and mandibular growth in the Late Jurassic docodontan Haldanodon exspectatus (Mammaliaformes)". Journal of Mammalian Evolution. 30 (3): 507–531. doi:10.1007/s10914-023-09668-2. S2CID 259535953.
- ^ Averianov, A. O.; Lopatin, A. V.; Leshchinskiy, S. V. (2023). "New interpretation of dentition in Early Cretaceous docodontan Sibirotherium based on micro-computed tomography". Journal of Mammalian Evolution. 30 (4): 811–817. doi:10.1007/s10914-023-09682-4. S2CID 261783889.
- ^ Sánchez-Beristain, F.; Rodrigo, J.; Schlagintweit, F. (2023). "Acanthochaetetes reitneri nov. sp. (Porifera: Demospongiae) from the Lower Cretaceous (upper Aptian–lower Albian) Tuburan Limestone of Cebu Island (Philippines) and its stratigraphic and palaeobiogeographic implications". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 307 (1): 17–28. doi:10.1127/njgpa/2023/1109. S2CID 256750180.
- ^ Luzhnaya, E. A.; Zhegallo, E. A.; Zaitseva, L. V.; Ragozina, A. L. (2023). "Problematic Porifera from the Lower Cambrian of Western Mongolia". Paleontological Journal. 57 (3): 256–269. Bibcode:2023PalJ...57..256L. doi:10.1134/S0031030123030103. S2CID 259336283.
- ^ Grimes, K. F.; Narbonne, G. M.; Gehling, J. G.; Trusler, P. W.; Dececchi, T. A. (2023). "Elongate Ediacaran fronds from the Flinders Ranges, South Australia". Journal of Paleontology. 98 (2): 249–265. doi:10.1017/jpa.2023.45. S2CID 261631797.
- ^ a b Peng, T.; Yang, Y.; Yun, H.; Yang, X.; Zhang, Q.; He, M.; Chi, X.; Liu, J.; Liu, X. (2023). "Flourishing chancelloriids from the Cambrian Kaili Biota of South China". Historical Biology: An International Journal of Paleobiology. 36 (7): 1302–1320. doi:10.1080/08912963.2023.2212382.
- ^ Chen, A.; Porras, L.; Ma, H.; Hou, X.; Wörheide, G. (2023). "A new sponge genus from the Chengjiang biota with an intriguing combination of skeletal characters". PalZ. 97 (3): 443–450. Bibcode:2023PalZ...97..443C. doi:10.1007/s12542-023-00652-y. S2CID 259791386.
- ^ a b Luo, C.; Poinar, G. O.; Xu, C.; Zhuo, D.; Jarzembowski, E. A.; Wang, B. (2023). "Widespread mermithid nematode parasitism of Cretaceous insects". eLife. 12. e86283. doi:10.7554/eLife.86283. PMC 10348742. PMID 37449724.
- ^ Botting, J. P.; Muir, L. A.; Doyle, E. (2023). "An oversized, late-surviving reticulosan sponge from the Carboniferous of Ireland". Geobios. 80: 1–13. Bibcode:2023Geobi..80....1B. doi:10.1016/j.geobios.2023.07.004. S2CID 260853505.
- ^ a b c Paulsen, Maria; Thibault, Nicolas (2023). "On the occurrence of rare nannoliths (calcareous nannofossils) in the Early Jurassic and their implications for the end-Triassic mass extinction". Papers in Palaeontology. 9 (2). Bibcode:2023PPal....9E1489P. doi:10.1002/spp2.1489. ISSN 2056-2799.
- ^ Wang, D.; Vannier, J.; Sun, J.; Yu, C.; Han, J. (2023). "A New Chengjiang Worm Sheds Light on the Radiation and Disparity in Early Priapulida". Biology. 12 (9). 1242. doi:10.3390/biology12091242. PMC 10525141. PMID 37759641.
- ^ Goñi, I.; Skovsted, C. B.; Li, L.; Li, G.; Betts, M. J.; Dorjnamjaa, D.; Altanshagai, G.; Enkhbaatar, B.; Topper, T. P. (2023). "New palaeoscolecid plates from the Cambrian Stage 3 of northern Mongolia". Acta Palaeontologica Polonica. 68 (1): 117–125. doi:10.4202/app.01030.2022. S2CID 256711586.
- ^ Zhao, J.; Li, Y.; Selden, P. A. (2023). "A new primitive polychaete with eyes from the lower Cambrian Guanshan biota of Yunnan Province, China". Frontiers in Ecology and Evolution. 11. 1128070. doi:10.3389/fevo.2023.1128070.
- ^ Davydov, A. E.; Yashunsky, Yu. V.; Mirantsev, G. V.; Krutykh, A. A. (2024). "New Hypercalcified Calcareous Sponges from the Gzhelian Stage of the Moscow Region". Paleontological Journal. 57 (11): 1325–1351. Bibcode:2024PalJ...57.1325D. doi:10.1134/S0031030123110035.
- ^ a b Zhang, Z.F.; Smith, M. R.; Ren, X.Y. (2023). "The Cambrian cirratuliform Iotuba denotes an early annelid radiation". Proceedings of the Royal Society B: Biological Sciences. 290 (1992). 20222014. doi:10.1098/rspb.2022.2014. PMC 9890102. PMID 36722078.
- ^ Świerczewska-Gładysz, E.; Jurkowska, A. (2023). "Taxonomy and palaeoecology of the Late Cretaceous (Campanian) Phymatellidae (lithistid demosponges) from the Miechów and Mogilno-Łódź synclinoria (southern and central Poland)" (PDF). Annales Societatis Geologorum Poloniae. 93 (3): 269–304. doi:10.14241/asgp.2023.03. S2CID 257661001.
- ^ Liu, Q.; Zong, R.; Li, Q.; Fang, X.; Huang, D. (2023). "New palaeoscolecidian worms from the Lower Ordovician Madaoyu Formation with specialised morphological characters and functional morphology". Historical Biology: An International Journal of Paleobiology. 36 (12): 2817–2828. doi:10.1080/08912963.2023.2278172. S2CID 265436470.
- ^ Botting, J. P.; Muir, L. A. (2023). "A new thalassematid echiuran worm from the Middle Ordovician Castle Bank Biota of Wales, UK". Acta Palaeontologica Polonica. 68 (4): 571–581. doi:10.4202/app.01107.2023.
- ^ Samant, B.; Pronzato, R.; Mohabey, D. M.; Cubeddu, T.; Stocchino, G. A.; Jangale, K.; Thalal, P.; Dhobale, A.; Manconi, R. (2023). "The oldest birotule-bearing freshwater sponges from the Upper Cretaceous–lower Paleocene Deccan volcanic-associated sediments of India". Acta Palaeontologica Polonica. 68 (1): 167–174. doi:10.4202/app.01040.2022. S2CID 257481832.
- ^ Nanglu, K.; Lerosey-Aubril, R.; Weaver, J. C.; Ortega-Hernández, J. (2023). "A mid-Cambrian tunicate and the deep origin of the ascidiacean body plan". Nature Communications. 14 (1). 3832. Bibcode:2023NatCo..14.3832N. doi:10.1038/s41467-023-39012-4. PMC 10325964. PMID 37414759.
- ^ McCall, C. R. A. (2023). "A large pelagic lobopodian from the Cambrian Pioche Shale of Nevada". Journal of Paleontology. 97 (5): 1009–1024. Bibcode:2023JPal...97.1009M. doi:10.1017/jpa.2023.63. S2CID 266292707.
- ^ Demidenko, Yu. E. (2023). "A new zooproblematic genus of the family Siphogonuchitidae". Paleontological Journal. 57 (3): 270–277. Bibcode:2023PalJ...57..270D. doi:10.1134/S003103012303005X. S2CID 259336505.
- ^ Kočí, T.; Milàn, J.; Jäger, M. (2023). "Neovermilia gundstrupensis sp. nov. (Polychaeta, Serpulidae) from the Selandian (middle Paleocene) of Fyn, Denmark". Bulletin of the Geological Society of Denmark. 72: 135–151. doi:10.37570/bgsd-2023-72-05. S2CID 259818185.
- ^ Wu, S.; Reitner, J.; Harper, D. A. T.; Yu, J.; Chen, Z.-Q. (2023). "New keratose sponges after the end-Permian extinction provide insights into biotic recoveries". Geobiology. 22 (1). e12582. doi:10.1111/gbi.12582. PMID 38385600. S2CID 266733934.
- ^ a b c Jeon, J.; Kershaw, S.; Liang, K.; Zhang, Y. (2023). "Stromatoporoids of the Katian (Upper Ordovician) Beiguoshan Formation, North China". Journal of Systematic Palaeontology. 21 (1). 2234929. Bibcode:2023JSPal..2134929J. doi:10.1080/14772019.2023.2234929. S2CID 261538981.
- ^ Wierzbowski, H.; Błażejowski, B. (2023). "Chaetognath grasping spines from the Devonian of Poland: their structure and geochemistry". Acta Palaeontologica Polonica. 68 (1): 103–116. doi:10.4202/app.01012.2022. S2CID 257420288.
- ^ a b Pisera, A.; Bitner, M. A.; Fromont, J. (2023). "Eocene phymaraphiniid demosponges from South Western Australia: filling the gap". Acta Palaeontologica Polonica. 68 (2): 261–272. doi:10.4202/app.01052.2023. S2CID 258601990.
- ^ Li, L.X.; Reitner, J. (2023). "A remarkable new halichondrid demosponge, Ptilospongia hemisphaeroidalis, from the latest Ordovician Beigong Biota, South China". Estonian Journal of Earth Sciences. 72 (1): 50–53. doi:10.3176/earth.2023.76. S2CID 259635250.
- ^ a b c McNamara, K. (2023). "The serpulid polychaete Rotulispira from the Late Cretaceous of Western Australia". Records of the Western Australian Museum. 38: 76–96. doi:10.18195/issn.0312-3162.38.2023.076-096.
- ^ Kimmig, J.; LaVine, R. J.; Schiffbauer, J. D.; Egenhoff, S. O.; Shelton, K. L.; Leibach, W. W. (2023). "Annelids from the Cambrian (Wuliuan Stage, Miaolingian) Spence Shale Lagerstätte of northern Utah, USA". Historical Biology: An International Journal of Paleobiology. 36 (5): 934–943. doi:10.1080/08912963.2023.2196685. PMC 11114447. PMID 38800616. S2CID 258047711.
- ^ Vinn, O.; Alkahtane, A. A.; El Hedeny, M. M.; Al Farraj, S.; Toom, U. (2023). "Earliest styliolinids from the Wenlock of Saaremaa Island (Estonia): paleoecological and evolutionary implications". Palaeoworld. 33 (4): 899–904. doi:10.1016/j.palwor.2023.09.004. S2CID 262207451.
- ^ Botting, J. P.; Muir, L. A.; Ma, J. (2023). "Teganium (Porifera, Hexactinellida) from the Middle Ordovician Castle Bank fauna of Avalonia (Wales, UK)". Palaeontologia Electronica. 26 (2). 26.2.21. doi:10.26879/1247.
- ^ Osawa, H.; Caron, J.-B.; Gaines, R. R. (2023). "First record of growth patterns in a Cambrian annelid". Royal Society Open Science. 10 (4). 221400. Bibcode:2023RSOS...1021400O. doi:10.1098/rsos.221400. PMC 10130728. PMID 37122950.
- ^ Muir, L. A.; Gutiérrez-Marco, J. C. (2023). "A new species of the problematic organism Webbyites from the Early Ordovician Fezouata Biota of Morocco". Estonian Journal of Earth Sciences. 72 (1): 74–77. doi:10.3176/earth.2023.24. hdl:10261/348447. S2CID 259573208.
- ^ Botha, T. L.; Sherratt, E.; Droser, M. L.; Gehling, J. G.; García-Bellido, D. C. (2023). "Elucidating the morphology and ecology of Eoandromeda octobrachiata from the Ediacaran of South Australia". Papers in Palaeontology. 9 (6). e1530. Bibcode:2023PPal....9E1530B. doi:10.1002/spp2.1530. hdl:2440/139912.
- ^ Darroch, S. A. F.; Gutarra, S.; Masaki, H.; Olaru, A.; Gibson, B. M.; Dunn, F. S.; Mitchell, E. G.; Racicot, R. A.; Burzynski, G.; Rahman, I. A. (2023). "The rangeomorph Pectinifrons abyssalis: hydrodynamic function at the dawn of animal life". iScience. 26 (2): 105989. Bibcode:2023iSci...26j5989D. doi:10.1016/j.isci.2023.105989. PMC 9900436. PMID 36756377. S2CID 256179963.
- ^ Pasinetti, G.; McIlroy, D. (2023). "Palaeobiology and taphonomy of the rangeomorph Culmofrons plumosa". Palaeontology. 66 (4). e12671. Bibcode:2023Palgy..6612671P. doi:10.1111/pala.12671. S2CID 260397981.
- ^ Retallack, G. J.; Matthews, N. A.; Master, S.; Khangar, R. G.; Khan, M. (2020). "Dickinsonia discovered in India and late Ediacaran biogeography". Gondwana Research. 90: 165–170. doi:10.1016/j.gr.2020.11.008. S2CID 229451488.
- ^ Meert, J. G.; Pandit, M. K.; Kwafo, S.; Singha, A. (2023). "Stinging News: 'Dickinsonia' discovered in the Upper Vindhyan of India Not Worth the Buzz". Gondwana Research. 117: 1–7. Bibcode:2023GondR.117....1M. doi:10.1016/j.gr.2023.01.003. S2CID 255846878.
- ^ Evans, S. D.; Hunt, G.; Gehling, J. G.; Sperling, E. A.; Droser, M. L. (2023). "Species of Dickinsonia Sprigg from the Ediacaran of South Australia". Palaeontology. 66 (1). e12635. Bibcode:2023Palgy..6612635E. doi:10.1111/pala.12635. S2CID 256673621.
- ^ Ivantsov, A. Y.; Zakrevskaya, M. (2023). "Body plan of Dickinsonia, the oldest mobile animals". Earth and Environmental Science Transactions of the Royal Society of Edinburgh. 114 (1–2): 95–108. Bibcode:2023EESTR.114...95I. doi:10.1017/S175569102300004X. S2CID 257168449.
- ^ Zhuravlev, A. Yu.; Wood, R. A.; Bowyer, F. T. (2023). "Cambrian radiation speciation events driven by sea level and redoxcline changes on the Siberian Craton". Science Advances. 9 (24): eadh2558. Bibcode:2023SciA....9H2558Z. doi:10.1126/sciadv.adh2558. PMC 10275598. PMID 37327332.
- ^ Gibson, B. M.; Chipman, M.; Attanasio, P.; Qureshi, Z.; Darroch, S. A. F.; Rahman, I. A.; Laflamme, M. (2023). "Reconstructing the feeding ecology of Cambrian sponge reefs: the case for active suspension feeding in Archaeocyatha". Royal Society Open Science. 10 (11). 230766. Bibcode:2023RSOS...1030766G. doi:10.1098/rsos.230766. PMC 10663785. PMID 38026009.
- ^ Yun, H.; Janussen, D.; Zhang, X.; Reitner, J. (2023). "Deep origin of the long root tuft: the oldest stalk-bearing sponge from the Cambrian Stage 3 black shale of South China". Lethaia. 56 (3): 1–10. Bibcode:2023Letha..56..3.5Y. doi:10.18261/let.56.3.5.
- ^ Łukowiak, M.; Meiro, G.; Peña, B.; Villanueva Guimerans, P.; Corbí, H. (2023). "Miocene sponge assemblages in the face of the Messinian Salinity Crisis—new data from the Atlanto-Mediterranean seaway". PeerJ. 11. e16277. doi:10.7717/peerj.16277. PMC 10657567. PMID 38025719.
- ^ Rogov, V.; Marusin, V.; Bykova, N.; Goy, Y.; Nagovitsin, K.; Kochnev, B.; Karlova, G.; Grazhdankin, D. (2012). "The oldest evidence of bioturbation on Earth". Geology. 40 (5): 395–398. Bibcode:2012Geo....40..395R. doi:10.1130/G32807.1.
- ^ Psarras, C.; Donoghue, P. C. J.; Garwood, R. J.; Grazhdankin, D. V.; Parry, L. A.; Rogov, V. I.; Liu, A. G. (2023). "Three-dimensional reconstruction, taphonomic and petrological data suggest that the oldest record of bioturbation is a body fossil coquina". Papers in Palaeontology. 9 (6). e1531. Bibcode:2023PPal....9E1531P. doi:10.1002/spp2.1531.
- ^ Yun, H.; Reitner, J.; Zhang, X. (2023). "Body reconstruction, taxonomy, and biostratigraphy of a 'problematic' chancelloriid". PalZ. 98: 29–40. doi:10.1007/s12542-023-00660-y. S2CID 260218700.
- ^ Yang, J.; Lan, T.; Zhang, X.; Smith, M. R. (2023). "Protomelission is an early dasyclad alga and not a Cambrian bryozoan". Nature. 615 (7952): 468–471. Bibcode:2023Natur.615..468Y. doi:10.1038/s41586-023-05775-5. PMID 36890226. S2CID 257425218.
- ^ Xiang, K.; Yin, Z.; Liu, W.; Zhao, F.; Zhu, M. (2023). "Early Cambrian Cambroclavus is a scleritomous eumetazoan unrelated to bryozoan or dasyclad algae". Geology. 52 (2): 130–134. doi:10.1130/G51663.1.
- ^ Zhao, Y.; Hou, X.-G.; Cong, P.-Y. (2023). "Tentacular nature of the 'column' of the Cambrian diploblastic Xianguangia sinica". Journal of Systematic Palaeontology. 21 (1). 2215787. Bibcode:2023JSPal..2115787Z. doi:10.1080/14772019.2023.2215787. S2CID 259400853.
- ^ Fatka, O.; Valent, M.; Budil, P. (2023). "The first healed injury in a hyolith operculum". The Science of Nature. 110 (5). 50. Bibcode:2023SciNa.110...50F. doi:10.1007/s00114-023-01879-0. PMID 37743430. S2CID 262218465.
- ^ Parry, L. A.; Edgecombe, G. D.; Bruthansová, J.; Vinther, J. (2023). "Healed injuries, ontogeny and scleritome construction in a Late Ordovician machaeridian (Annelida, Aphroditiformia)". Papers in Palaeontology. 9 (4). e1520. Bibcode:2023PPal....9E1520P. doi:10.1002/spp2.1520. S2CID 261130565.
- ^ Gueriau, P.; Parry, L. A.; Rabet, N. (2023). "Gilsonicaris from the Lower Devonian Hunsrück slate is a eunicidan annelid and not the oldest crown anostracan crustacean". Biology Letters. 19 (8). 20230312. doi:10.1098/rsbl.2023.0312. PMC 10465189. PMID 37643644.
- ^ Zhang, H.; Xiao, S.; Eriksson, M. E.; Duan, B.; Maas, A. (2023). "Musculature of an Early Cambrian cycloneuralian animal". Proceedings of the Royal Society B: Biological Sciences. 290 (2008). 20231803. doi:10.1098/rspb.2023.1803. PMC 10565385. PMID 37817588.
- ^ Braddy, S. J.; Gass, K. C.; Tessler, M. (2023). "Not the first leech: An unusual worm from the early Silurian of Wisconsin". Journal of Paleontology. 97 (4): 799–804. Bibcode:2023JPal...97..799B. doi:10.1017/jpa.2023.47. S2CID 261535626.
- ^ Legg, D. A.; Sutton, M. D.; Edgecombe, G. D. (2013). "Arthropod fossil data increase congruence of morphological and molecular phylogenies". Nature Communications. 4. 2485. Bibcode:2013NatCo...4.2485L. doi:10.1038/ncomms3485. PMID 24077329.
- ^ Yang, J.; Ortega-Hernández, J.; Butterfield, N. J.; Liu, Y.; Boyan, G. S.; Hou, J.; Lan, T.; Zhang, X. (2016). "Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda". Proceedings of the National Academy of Sciences of the United States of America. 113 (11): 2988–2993. Bibcode:2016PNAS..113.2988Y. doi:10.1073/pnas.1522434113. PMC 4801254. PMID 26933218.
- ^ Aria, C.; Zhao, F.; Zhu, M. (2021). "Fuxianhuiids are mandibulates and share affinities with total-group Myriapoda". Journal of the Geological Society. 178 (5): jgs2020-246. Bibcode:2021JGSoc.178..246A. doi:10.1144/jgs2020-246. S2CID 233952670.
- ^ Wu, R.; Pisani, D.; Donoghue, I. M. (2023). "The unbearable uncertainty of panarthropod relationships". Biology Letters. 19 (1). 20220497. doi:10.1098/rsbl.2022.0497. PMC 9832341. PMID 36628953.
- ^ Kihm, J.-H.; Smith, F. W.; Kim, S.; Rho, H. S.; Zhang, X.; Liu, J.; Park, T.-Y. S. (2023). "Cambrian lobopodians shed light on the origin of the tardigrade body plan". Proceedings of the National Academy of Sciences of the United States of America. 120 (28): e2211251120. Bibcode:2023PNAS..12011251K. doi:10.1073/pnas.2211251120. PMC 10334802. PMID 37399417.
- ^ Li, Y.; Dunn, F. S.; Murdock, D. J. E.; Guo, J.; Rahman, I. A.; Cong, P. (2023). "Cambrian stem-group ambulacrarians and the nature of the ancestral deuterostome". Current Biology. 33 (12): 2359–2366.e2. Bibcode:2023CBio...33E2359L. doi:10.1016/j.cub.2023.04.048. PMID 37167976. S2CID 258592223.
- ^ Yang, Y.; Su, B.; Ou, Q.; Cheng, M.; Han, J.; Shu, D. (2023). "An enigmatic structure in the tail of vetulicolians from the Cambrian Chengjiang biota, South China". Papers in Palaeontology. 9 (6). e1537. Bibcode:2023PPal....9E1537Y. doi:10.1002/spp2.1537. S2CID 265626249.
- ^ Yang, X.; Kimmig, J.; Schiffbauer, J. D.; Peng, S. (2023). "Herpetogaster collinsi from the Cambrian of China elucidates the dispersal and palaeogeographic distribution of early deuterostomes and the origin of the ambulacrarian larva". PeerJ. 11. e16385. doi:10.7717/peerj.16385. PMC 10637255. PMID 37953779.
- ^ Tian, Q.; Zhao, F.; Zeng, H.; Zhu, M.; Jiang, B. (2022). "Ultrastructure reveals ancestral vertebrate pharyngeal skeleton in yunnanozoans". Science. 377 (6602): 218–222. Bibcode:2022Sci...377..218T. doi:10.1126/science.abm2708. PMID 35857544. S2CID 250380981.
- ^ He, K.; Liu, J.; Han, J.; Ou, Q.; Chen, A.; Zhang, Z.; Fu, D.; Hua, H.; Zhang, X.; Shu, D. (2023). "Comment on "Ultrastructure reveals ancestral vertebrate pharyngeal skeleton in yunnanozoans"". Science. 381 (6656). eade9707. doi:10.1126/science.ade9707. PMID 37499008. S2CID 260202685.
- ^ Zhang, X.-G.; Pratt, B. R. (2023). "Comment on "Ultrastructure reveals ancestral vertebrate pharyngeal skeleton in yunnanozoans"". Science. 381 (6656). eadf1472. doi:10.1126/science.adf1472. PMID 37498997. S2CID 260202683.
- ^ Tian, Q.; Zhao, F.; Zeng, H.; Zhu, M.; Jiang, B. (2023). "Response to Comments on "Ultrastructure reveals ancestral vertebrate pharyngeal skeleton in yunnanozoans"". Science. 381 (6656). eadf3363. doi:10.1126/science.adf3363. PMID 37499010. S2CID 260202688.
- ^ Jenkins, K. M.; Meyer, D. L.; Bhullar, B.-A. S. (2023). "Lost and Found: Redescription of Chamasaurus dolichognathus Williston 1915 from the Permo-Carboniferous of New Mexico". Bulletin of the Peabody Museum of Natural History. 64 (1): 3–9. doi:10.3374/014.064.0101. S2CID 257755687.
- ^ Mikami, T.; Ikeda, T.; Muramiya, Y.; Hirasawa, T.; Iwasaki, W. (2023). "Three-dimensional anatomy of the Tully monster casts doubt on its presumed vertebrate affinities". Palaeontology. 66 (2). e12646. Bibcode:2023Palgy..6612646M. doi:10.1111/pala.12646. S2CID 258198566.
- ^ a b c d Ghavidel-Syooki, M.; Piri-Kangarshahi, H. H. (2023). "Peri-Gondwanan acritarchs and chitinozoans from the Lower–Middle Ordovician Lashkarak Formation in the Alborz Mountain Ranges, northern Iran: regional stratigraphical significance and palaeogeographical implications". Palynology. 47 (3). 2214191. Bibcode:2023Paly...4714191G. doi:10.1080/01916122.2023.2214191. S2CID 258737255.
- ^ a b c d e Riedman, L. A.; Porter, S. M.; Lechte, M. A.; dos Santos, A.; Halverson, G. P. (2023). "Early eukaryotic microfossils of the late Palaeoproterozoic Limbunya Group, Birrindudu Basin, northern Australia". Papers in Palaeontology. 9 (6). e1538. Bibcode:2023PPal....9E1538R. doi:10.1002/spp2.1538.
- ^ Peel, J. S. (2023). "A phosphatised fossil Lagerstätte from the middle Cambrian (Wuliuan Stage) of North Greenland (Laurentia)". Bulletin of the Geological Society of Denmark. 72: 102–122. doi:10.37570/bgsd-2023-72-03. S2CID 259807172.
- ^ Wang, K.; Xu, H.-H.; Liu, B.-C.; Bai, J.; Wang, Y.; Tang, P.; Lu, J.-F.; Wang, Y. (2023). "Shallow-marine testate amoebae with internal structures from the Lower Devonian of China". iScience. 26 (5): 106678. Bibcode:2023iSci...26j6678W. doi:10.1016/j.isci.2023.106678. PMC 10173733. PMID 37182111.
- ^ Liu, W.; Yin, Z.; Pan, B.; Shen, B.; Dong, L.; Li, G. (2023). "Germ-soma differentiation and reproduction in a new species of early Cambrian acritarch". Palaeontology. 66 (6). e12687. Bibcode:2023Palgy..6612687L. doi:10.1111/pala.12687. S2CID 266552287.
- ^ a b c Xian, X.; Eriksson, M. E.; Zhang, H. (2023). "Reassessment of Archaeooides based on new material from the Fortunian (early Cambrian) of China infers algal affinity". Palaeogeography, Palaeoclimatology, Palaeoecology. 637. 112011. doi:10.1016/j.palaeo.2023.112011.
- ^ a b c Chen, K.; Miao, L.; Zhao, F.; Zhu, M. (2023). "Carbonaceous macrofossils from the early Mesoproterozoic Gaoyuzhuang Formation in the Yanshan Range, North China". Precambrian Research. 392. 107074. Bibcode:2023PreR..39207074C. doi:10.1016/j.precamres.2023.107074. S2CID 258675790.
- ^ Vorob'eva, N. G.; Petrov, P. Yu. (2023). "Middle Ura Association of Organic-Walled Microfossils: the Lower Vendian of the Patom Basin, Siberia". Stratigraphy and Geological Correlation. 31 (5): 410–424. Bibcode:2023SGC....31..410V. doi:10.1134/S086959382305009X.
- ^ a b Peng, J.; Slater, S. M.; McLoughlin, S.; Vajda, V. (2023). "New species of Kuqaia from the Lower Jurassic of Sweden indicates a possible water flea (Crustacea: Branchiopoda) affinity". PLOS ONE. 18 (6). e0282247. Bibcode:2023PLoSO..1882247P. doi:10.1371/journal.pone.0282247. PMC 10246822. PMID 37285340.
- ^ Van Damme, K. (2023). "Identification of ancient Cladocera-like fossils requires homologies: The Jurassic Kuqaia is not a Waterflea". Zootaxa. 5343 (1): 91–97. doi:10.11646/zootaxa.5343.1.6. PMID 38221385. S2CID 261576944.
- ^ a b Kaczmarska, I.; Ehrman, J. M. (2023). "Parmalean and other siliceous nannofossils from the Oligocene of Polish Flysch Carpathians". Acta Palaeontologica Polonica. 68 (3): 441–456. doi:10.4202/app.01078.2023.
- ^ Skompski, S.; Kozłowska, A.; Kozłowski, W.; Łuczyński, P. (2023). "Coexistence of algae and a graptolite-like problematicum: a case study from the late Silurian of Podolia (Ukraine)". Acta Geologica Polonica. 73 (2): 115–133. doi:10.24425/agp.2022.143599. S2CID 259642886.
- ^ LoDuca, S. T. (2024). "Reinterpretation of Voronocladus from the Silurian of Ukraine as a bryopsidalean alga (Chlorophyta): The outlines of a major early Paleozoic macroalgal radiation begin to come into focus". Review of Palaeobotany and Palynology. 322. 105064. Bibcode:2024RPaPa.32205064L. doi:10.1016/j.revpalbo.2024.105064. S2CID 267155829.
- ^ Martyshyn, A. I. (2023). "Tymkivia primitiva gen. nov. sp. nov., a new type of fossils from the Late Ediacaran (Vendian) Kanylivka Group in Podolia, Ukraine". Geologičnij žurnal. 2023 (2): 58–67. doi:10.30836/igs.1025-6814.2023.2.275044. S2CID 259549401.
- ^ Tang, D.-J.; Shi, X.-Y.; Zhou, X.-Q.; Riding, R. (2023). "Mesoproterozoic biomineralization: Cyanobacterium-like filamentous siderite sheaths ~1.4 Ga". Journal of Palaeogeography. 12 (3): 384–400. Bibcode:2023JPalG..12..384T. doi:10.1016/j.jop.2023.03.006. S2CID 257988659.
- ^ Ngwal'ghoubou Ikouanga, J.; Fontaine, C.; Bourdelle, F.; Abd Elmola, A.; Aubineau, J.; Bankole, O. M.; Reisberg, L.; Pierson-Wickmann, A.-C.; Riboulleau, A.; Trentesaux, A.; Laforest, C.; Meunier, A.; El Albani, A. (2023). "Taphonomy of early life (2.1 Ga) in the francevillian basin (Gabon): Role of organic mineral interactions" (PDF). Precambrian Research. 395: 107155. Bibcode:2023PreR..39507155I. doi:10.1016/j.precamres.2023.107155. S2CID 260400276.
- ^ Franz, G.; Khomenko, V.; Lyckberg, P.; Chournousenko, V.; Struck, U.; Gernert, U.; Nissen, J. (2023). "The Volyn biota (Ukraine) – indications of 1.5 Gyr old eukaryotes in 3D preservation, a spotlight on the "boring billion"". Biogeosciences. 20 (10): 1901–1924. Bibcode:2023BGeo...20.1901F. doi:10.5194/bg-20-1901-2023. S2CID 258899338.
- ^ Hoshino, Y.; Nettersheim, B. J.; Gold, D. A.; Hallmann, C.; Vinnichenko, G.; van Maldegem, L. M.; Bishop, C.; Brocks, J. J.; Gaucher, E. A. (2023). "Genetics re-establish the utility of 2-methylhopanes as cyanobacterial biomarkers before 750 million years ago". Nature Ecology & Evolution. 7 (12): 2045–2054. Bibcode:2023NatEE...7.2045H. doi:10.1038/s41559-023-02223-5. PMC 10697835. PMID 37884688.
- ^ Strullu-Derrien, C.; Fercoq, F.; Gèze, M.; Kenrick, P.; Martos, F.; Selosse, M.-A.; Benzerara, K.; Knoll, A. H. (2023). "Hapalosiphonacean cyanobacteria (Nostocales) thrived amid emerging embryophytes in an Early Devonian (407-million-year-old) landscape". iScience. 26 (8): 107338. Bibcode:2023iSci...26j7338S. doi:10.1016/j.isci.2023.107338. PMC 10382934. PMID 37520734. S2CID 259666124.
- ^ Li, G.; Chen, L.; Pang, K.; Tang, Q.; Wu, C.; Yuan, X.; Zhou, C.; Xiao, S. (2023). "Tonian carbonaceous compressions indicate that Horodyskia is one of the oldest multicellular and coenocytic macro-organisms". Communications Biology. 6 (1). 399. doi:10.1038/s42003-023-04740-2. PMC 10097871. PMID 37046079.
- ^ Li, G.; Pang, K.; Tang, Q.; Chen, L.; Wu, C.; Huang, R.; Wan, B.; Yuan, X.; Zhou, C. (2023). "Tonian discoidal fossils from North China: Relating discs to worm-like annulated tubes and their paleoecological and evolutionary implications". Palaeogeography, Palaeoclimatology, Palaeoecology. 628. 111780. Bibcode:2023PPP...62811780L. doi:10.1016/j.palaeo.2023.111780. S2CID 261036865.
- ^ Barling, N.; Saleh, F.; Ma, X. (2023). "A unique record of prokaryote cell pyritization". Geology. 51 (11): 1062–1066. Bibcode:2023Geo....51.1062B. doi:10.1130/G51352.1. S2CID 261786690.
- ^ Bryłka, K.; Alverson, A. J.; Pickering, R. A.; Richoz, S.; Conley, D. J. (2023). "Uncertainties surrounding the oldest fossil record of diatoms". Scientific Reports. 13 (1). 8047. Bibcode:2023NatSR..13.8047B. doi:10.1038/s41598-023-35078-8. PMC 10192206. PMID 37198388.
- ^ Ciurej, A.; Dubicka, Z.; Poberezhskyy, A. (2023). "Calcareous dinoflagellate blooms during the Late Cretaceous 'greenhouse' world—a case study from western Ukraine". PeerJ. 11. e16201. doi:10.7717/peerj.16201. PMC 10560496. PMID 37814625.
- ^ Bryant, R.; Meehan, K. C.; Belanger, C. L. (2023). "Are ancient seep environments distinguishable by benthic foraminiferal assemblages? A case study of the Cretaceous Western Interior Seaway". Cretaceous Research. 146. 105476. Bibcode:2023CrRes.14605476B. doi:10.1016/j.cretres.2023.105476. S2CID 256173904.
- ^ Fenton, I. S.; Aze, T.; Farnsworth, A.; Valdes, P.; Saupe, E. E. (2023). "Origination of the modern-style diversity gradient 15 million years ago". Nature. 614 (7949): 708–712. Bibcode:2023Natur.614..708F. doi:10.1038/s41586-023-05712-6. PMID 36792825. S2CID 256899993. Archived from the original on 20 Mar 2023.
- ^ Woodhouse, A.; Swain, A.; Fagan, W. F.; Fraass, A. J.; Lowery, C. M. (2023). "Late Cenozoic cooling restructured global marine plankton communities". Nature. 614 (7949): 713–718. Bibcode:2023Natur.614..713W. doi:10.1038/s41586-023-05694-5. PMID 36792824. S2CID 253282752.
- ^ Fonseca, C.; Mendonça Filho, J. G.; Reolid, M.; Duarte, L. V.; Oliveira, A. D.; Souza, J. T.; Lézin, C. (2023). "First putative occurrence in the fossil record of choanoflagellates, the sister group of Metazoa". Scientific Reports. 13 (1). 1242. Bibcode:2023NatSR..13.1242F. doi:10.1038/s41598-022-26972-8. PMC 9870899. PMID 36690681.
- ^ Brocks, J. J.; Nettersheim, B. J.; Adam, P.; Schaeffer, P.; Jarrett, A. J. M.; Güneli, N.; Liyanage, T.; van Maldegem, L. M.; Hallmann, C.; Hope, J. M. (2023). "Lost world of complex life and the late rise of the eukaryotic crown" (PDF). Nature. 618 (7966): 767–773. Bibcode:2023Natur.618..767B. doi:10.1038/s41586-023-06170-w. PMID 37286610. S2CID 259111647.
- ^ Choudhuri, A.; El Albani, A.; Mandal, S.; Sarkar, S. (2023). "Biotic vs abiotic origin of unusual features from Mesoproterozoic of Vindhyan Supergroup, India". Annales de Paléontologie. 109 (3). 102629. Bibcode:2023AnPal.10902629C. doi:10.1016/j.annpal.2023.102629. S2CID 262177536.
- ^ Wood, R.; Bowyer, F. T.; Alexander, R.; Yilales, M.; Uahengo, C.-I.; Kaputuaza, K.; Ndeunyema, J.; Curtis, A. (2023). "New Ediacaran biota from the oldest Nama Group, Namibia (Tsaus Mountains), and re-definition of the Nama Assemblage". Geological Magazine. 160 (9): 1673–1686. Bibcode:2023GeoM..160.1673W. doi:10.1017/S0016756823000638. hdl:20.500.11820/bc8c23b0-d59c-4230-a45b-db854a8ad0f3.
- ^ Bowyer, F. T.; Uahengo, C.-I.; Kaputuaza, K.; Ndeunyema, J.; Yilales, M.; Alexander, R. D.; Curtis, A.; Wood, R. A. (2023). "Constraining the onset and environmental setting of metazoan biomineralization: The Ediacaran Nama Group of the Tsaus Mountains, Namibia". Earth and Planetary Science Letters. 620. 118336. Bibcode:2023E&PSL.62018336B. doi:10.1016/j.epsl.2023.118336.
- ^ Kolesnikov, A. V.; Latysheva, I. V.; Shatsillo, A. V.; Kuznetsov, N. B.; Kolesnikov, A. S.; Desiatkin, V. D.; Romanyuk, T. V. (2023). "Ediacara-Type Biota in the Upper Precambrian of the Timan Range (Dzhezhim–Parma Hill, Komi Republic)". Doklady Earth Sciences. 510 (1): 289–292. Bibcode:2023DokES.510..289K. doi:10.1134/S1028334X23600032. S2CID 258470518.
- ^ Petrov, P. Yu.; Vorob'eva, N. G. (2023). "Fossils, pseudofossils and problematica from the Ura Formation: Ediacaran of the Patom Basin, Siberia". Precambrian Research. 397. 107188. Bibcode:2023PreR..39707188Y. doi:10.1016/j.precamres.2023.107188. S2CID 262228437.
- ^ Mussini, G.; Dunn, F. S. (2023). "Decline and fall of the Ediacarans: late-Neoproterozoic extinctions and the rise of the modern biosphere". Biological Reviews. 99 (1): 110–130. doi:10.1111/brv.13014. PMID 37667585.
- ^ Servais, T.; Cascales-Miñana, B.; Harper, D. A. T.; Lefebvre, B.; Munnecke, A.; Wang, W.; Zhang, Y. (2023). "No (Cambrian) explosion and no (Ordovician) event: A single long-term radiation in the early Palaeozoic". Palaeogeography, Palaeoclimatology, Palaeoecology. 623. 111592. Bibcode:2023PPP...62311592S. doi:10.1016/j.palaeo.2023.111592. S2CID 258371439.
- ^ Pohl, A.; Stockey, R. G.; Dai, X.; Yohler, R.; Le Hir, G.; Hülse, D.; Brayard, A.; Finnegan, S.; Ridgwell, A. (2023). "Why the Early Paleozoic was intrinsically prone to marine extinction". Science Advances. 9 (35): eadg7679. Bibcode:2023SciA....9G7679P. doi:10.1126/sciadv.adg7679. PMC 10468122. PMID 37647393.
- ^ Monarrez, P. M.; Heim, N. A.; Payne, J. L. (2023). "Reduced strength and increased variability of extinction selectivity during mass extinctions". Royal Society Open Science. 10 (9). 230795. Bibcode:2023RSOS...1030795M. doi:10.1098/rsos.230795. PMC 10523066. PMID 37771968.
- ^ Høyberget, M.; Ebbestad, J. O. R.; Funke, B.; Funke, M.-L. K.; Nakrem, H. A. (2023). "The Skyberg Lagerstätte from the Mjøsa area, Norway: a rare window into the late early Cambrian biodiversity of Scandinavia". Lethaia. 56 (2): 1–28. Bibcode:2023Letha..56..2.4H. doi:10.18261/let.56.2.4. S2CID 258469759.
- ^ Li, L.; Betts, M. J.; Yun, H.; Pan, B.; Topper, T. P.; Li, G.; Zhang, X.; Skovsted, C. B. (2023). "Fibrous or Prismatic? A Comparison of the Lamello-Fibrillar Nacre in Early Cambrian and Modern Lophotrochozoans". Biology. 12 (1). 113. doi:10.3390/biology12010113. PMC 9855346. PMID 36671805.
- ^ Du, M.; Li, H.; Tan, J.; Wang, Z.; Wang, W. (2023). "The bias types and drivers of the Furongian Biodiversity Gap". Palaeogeography, Palaeoclimatology, Palaeoecology. 612. 111394. Bibcode:2023PPP...61211394D. doi:10.1016/j.palaeo.2023.111394. S2CID 255717556.
- ^ Eliahou Ontiveros, D.; Beaugrand, G.; Lefebvre, B.; Marcilly, C. M.; Servais, T.; Pohl, A. (2023). "Impact of global climate cooling on Ordovician marine biodiversity". Nature Communications. 14 (1). 6098. Bibcode:2023NatCo..14.6098O. doi:10.1038/s41467-023-41685-w. PMC 10564867. PMID 37816739.
- ^ Botting, J. P.; Muir, L. A.; Pates, S.; McCobb, L. M. E.; Wallet, E.; Willman, S.; Zhang, Y.; Ma, J. (2023). "A Middle Ordovician Burgess Shale-type fauna from Castle Bank, Wales (UK)". Nature Ecology & Evolution. 7 (5): 666–674. Bibcode:2023NatEE...7..666B. doi:10.1038/s41559-023-02038-4. PMID 37127766. S2CID 258438453.
- ^ Majchrzyk, A.; Jakubowicz, M.; Berkowski, B.; Król, J. J.; Zatoń, M.; Zapalski, M. K. (2023). "Modern-type reef in ancient time - Palaeoecology of a Middle Devonian coral community from Madène el Mrakib (Anti-Atlas, Morocco)". Palaeogeography, Palaeoclimatology, Palaeoecology. 633. 111876. doi:10.1016/j.palaeo.2023.111876.
- ^ Guo, X.; Retallack, G. J.; Liu, J. (2023). "Paleoenvironments of Late Devonian tetrapods in China". Scientific Reports. 13 (1). 20378. Bibcode:2023NatSR..1320378G. doi:10.1038/s41598-023-47728-y. PMC 10663569. PMID 37990036.
- ^ Dunne, E. M.; Thompson, S. E. D.; Butler, R. J.; Rosindell, J.; Close, R. A. (2023). "Mechanistic neutral models show that sampling biases drive the apparent explosion of early tetrapod diversity". Nature Ecology & Evolution. 7 (9): 1480–1489. Bibcode:2023NatEE...7.1480D. doi:10.1038/s41559-023-02128-3. PMC 10482683. PMID 37500908.
- ^ Francischini, H.; Dentzien-Dias, P.; Battista, F.; Sipp, G. S.; Melo, T. P.; Scherer, C. M. S.; Schultz, C. L. (2023). "Burrows provided shelter for tetrapods in a Permo-Triassic desert". Papers in Palaeontology. 9 (2). e1490. Bibcode:2023PPal....9E1490F. doi:10.1002/spp2.1490. S2CID 258252228.
- ^ Huang, Y.; Chen, Z.-Q.; Roopnarine, P. D.; Benton, M. J.; Zhao, L.; Feng, X.; Li, Z. (2023). "The stability and collapse of marine ecosystems during the Permian-Triassic mass extinction". Current Biology. 33 (6): 1059–1070.e4. Bibcode:2023CBio...33E1059H. doi:10.1016/j.cub.2023.02.007. PMID 36841237. S2CID 257186215.
- ^ Kelley, B. M.; Yu, M.; Lehrmann, D. J.; Altıner, D.; Payne, J. L. (2023). "Prolonged and gradual recovery of metazoan-algal reefs following the end-Permian mass extinction". Geology. doi:10.1130/G51058.1. S2CID 260913735.
- ^ Dai, X.; Davies, J. H. F. L.; Yuan, Z.; Brayard, A.; Ovtcharova, M.; Xu, G.; Liu, X.; Smith, C. P. A.; Schweitzer, C. E.; Li, M.; Perrot, M. G.; Jiang, S.; Miao, L.; Cao, Y.; Yan, J.; Bai, R.; Wang, F.; Guo, W.; Song, H.; Tian, L.; Dal Corso, J.; Liu, Y.; Chu, D.; Song, H. (2023). "A Mesozoic fossil lagerstätte from 250.8 million years ago shows a modern-type marine ecosystem". Science. 379 (6632): 567–572. Bibcode:2023Sci...379..567D. doi:10.1126/science.adf1622. PMID 36758082. S2CID 256697946.
- ^ Czepiński, Ł.; Pawlak, W.; Rytel, A.; Tałanda, M.; Szczygielski, T.; Sulej, T. (2023). "A new Middle Triassic vertebrate assemblage from Miedary (southern Poland". Journal of Vertebrate Paleontology. 43 (2). e2265445. doi:10.1080/02724634.2023.2265445. S2CID 266965172.
- ^ Lukeneder, A.; Lukeneder, P. (2023). "New data on the marine Upper Triassic palaeobiota from the Polzberg Konservat-Lagerstätte in Austria". Swiss Journal of Palaeontology. 142 (1). 9. Bibcode:2023SwJP..142....9L. doi:10.1186/s13358-023-00269-3.
- ^ Cribb, A. T.; Formoso, K. K.; Woolley, C. H.; Beech, J.; Brophy, S.; Byrne, P.; Cassady, V. C.; Godbold, A. L.; Larina, E.; Maxeiner, P.; Wu, Y.-H.; Corsetti, F. A.; Bottjer, D. J. (2023). "Contrasting terrestrial and marine ecospace dynamics after the end-Triassic mass extinction event". Proceedings of the Royal Society B: Biological Sciences. 290 (2012). 20232232. doi:10.1098/rspb.2023.2232. PMC 10697803. PMID 38052241.
- ^ El Atfy, H.; Abeed, Q.; Uhl, D. (2023). "Non-pollen palynomorph and palynofacies assemblages from the Lower Cretaceous of Iraq: A glimpse into palaeobiology and palaeoenvironment". Geodiversitas. 45 (11): 353–366. doi:10.5252/geodiversitas2023v45a11. S2CID 259275507.
- ^ Del Mouro, L.; Becker-Kerber, B.; Janasi, V. A.; Carvalho, M. A.; Waichel, B. L.; Lima, E. F.; Rossetti, L. M. M.; Cruz, V.; Silva, M. S.; Famelli, N.; Ortega-Hernández, J. (2023). "Organic walled microfossils in wet peperites from the early Cretaceous Paraná-Etendeka volcanism of Brazil". Scientific Reports. 13 (1). 15362. Bibcode:2023NatSR..1315362D. doi:10.1038/s41598-023-42483-6. PMC 10505181. PMID 37717103.
- ^ Cortés, D.; Larsson, H. C. E. (2023). "Top of the food chains: an ecological network of the marine Paja Formation biota from the Early Cretaceous of Colombia reveals the highest trophic levels ever estimated". Zoological Journal of the Linnean Society. 202. doi:10.1093/zoolinnean/zlad092.
- ^ Purcell, C.; Scuderi, L.; Myers, C. (2023). "Faunal provinciality in the Late Cretaceous Western Interior Seaway using network modeling". Geology. 51 (9): 839–844. Bibcode:2023Geo....51..839P. doi:10.1130/G51255.1. S2CID 259649890.
- ^ Jambura, P. L.; Solonin, S. V.; Cooper, S. L. A.; Mychko, E. V.; Arkhangelsky, M. S.; Türtscher, J.; Amadori, M.; Stumpf, S.; Vodorezov, A. V.; Kriwet, J. (2023). "Fossil marine vertebrates (Chondrichthyes, Actinopterygii, Reptilia) from the Upper Cretaceous of Akkermanovka (Orenburg Oblast, Southern Urals, Russia)". Cretaceous Research. 155. 105779. doi:10.1016/j.cretres.2023.105779. PMC 7615991. PMID 38799703.
- ^ Bobe, R.; Aldeias, V.; Alemseged, Z.; Anemone, R. L.; Archer, W.; Aumaître, G.; Bamford, M. K.; Biro, D.; Bourlès, D. L.; Boyd, M. D.; Braun, D. R.; Capelli, C.; d'Oliveira Coelho, J.; Habermann, J. M.; Head, J. J.; Keddadouche, K.; Kupczik, K.; Lebatard, A.-E.; Lüdecke, T.; Macôa, A.; Martínez, F. I.; Mathe, J.; Mendes, C.; Paulo, L. M.; Pinto, M.; Presnyakova, D.; Püschel, T. A.; Regala, F. T.; Sier, M.; Ferreira da Silva, M. J.; Stalmans, M.; Carvalho, S. (2023). "The first Miocene fossils from coastal woodlands in the southern East African Rift". iScience. 26 (9). 107644. Bibcode:2023iSci...26j7644B. doi:10.1016/j.isci.2023.107644. PMC 10494320. PMID 37701811. S2CID 260949644.
- ^ Hayward, Bruce W.; Stolberger, Thomas F.; Collins, Nathan; Beu, Alan G.; Blom, Wilma (27 August 2023). "A diverse Late Pliocene fossil fauna and its paleoenvironment at Māngere, Auckland, New Zealand". New Zealand Journal of Geology and Geophysics. doi:10.1080/00288306.2023.2243234. ISSN 0028-8306. S2CID 261264121. Wikidata Q123698788.
- ^ Harrison, T.; Su, D. F.; Fillion, E. N.; Kwekason, A. (2023). "Early Pliocene fauna from the Lower Laetolil Beds, Laetoli, Tanzania". Historical Biology: An International Journal of Paleobiology. 36 (11): 2386–2417. doi:10.1080/08912963.2023.2258907. S2CID 263243240.
- ^ Rozas-Davila, A.; Rodbell, D. T.; Bush, M. B. (2023). "Pleistocene megafaunal extinction in the grasslands of Junín-Peru". Journal of Biogeography. 50 (4): 755–766. Bibcode:2023JBiog..50..755R. doi:10.1111/jbi.14566. S2CID 256255790.
- ^ Martinez, Q.; Okrouhlík, J.; Šumbera, R.; Wright, M.; Araújo, R.; Braude, S.; Hildebrandt, T. B.; Holtze, S.; Ruf, I.; Fabre, P.-H. (2023). "Mammalian maxilloturbinal evolution does not reflect thermal biology". Nature Communications. 14 (1). 4425. Bibcode:2023NatCo..14.4425M. doi:10.1038/s41467-023-39994-1. PMC 10361988. PMID 37479710.
- ^ Bowyer, F. T.; Krause, A. J.; Song, Y.; Huang, K.-J.; Fu, Y.; Shen, B.; Li, J.; Zhu, X.-K.; Kipp, M. A.; van Maldegem, L. M.; Brocks, J. J.; Shields, G. A.; Le Hir, G.; Mills, B. J. W.; Poulton, S. W. (2023). "Biological diversification linked to environmental stabilization following the Sturtian Snowball glaciation". Science Advances. 9 (34): eadf9999. Bibcode:2023SciA....9F9999B. doi:10.1126/sciadv.adf9999. PMC 10456883. PMID 37624887.
- ^ Song, H.; An, Z.; Ye, Q.; Stüeken, E. E.; Li, J.; Hu, J.; Algeo, T. J.; Tian, L.; Chu, D.; Song, H.; Xiao, S.; Tong, J. (2023). "Mid-latitudinal habitable environment for marine eukaryotes during the waning stage of the Marinoan snowball glaciation". Nature Communications. 14 (1). 1564. doi:10.1038/s41467-023-37172-x. PMC 10073137. PMID 37015913.
- ^ Wang, X.; Algeo, T. J.; Li, C.; Zhu, M. (2023). "Spatial pattern of marine oxygenation set by tectonic and ecological drivers over the Phanerozoic". Nature Geoscience. 16 (11): 1020–1026. Bibcode:2023NatGe..16.1020W. doi:10.1038/s41561-023-01296-y. S2CID 264525407.
- ^ Bowyer, F. T.; Zhuravlev, A. Yu; Wood, R.; Zhao, F.; Sukhov, S. S.; Alexander, R. D.; Poulton, S. W.; Zhu, M. (2023). "Implications of an integrated late Ediacaran to early Cambrian stratigraphy of the Siberian Platform, Russia" (PDF). GSA Bulletin. doi:10.1130/B36534.1. S2CID 255892722.
- ^ Salles, T.; Husson, L.; Lorcery, M.; Halder Boggiani, B. (2023). "Landscape dynamics and the Phanerozoic diversification of the biosphere". Nature. 624 (7990): 115–121. Bibcode:2023Natur.624..115S. doi:10.1038/s41586-023-06777-z. PMC 10700141. PMID 38030724.
- ^ Nelson, L. L.; Crowley, J. L.; Smith, E. F.; Schwartz, D. M.; Hodgin, E. B.; Schmitz, M. D. (2023). "Cambrian explosion condensed: High-precision geochronology of the lower Wood Canyon Formation, Nevada". Proceedings of the National Academy of Sciences of the United States of America. 120 (30). e2301478120. Bibcode:2023PNAS..12001478N. doi:10.1073/pnas.2301478120. PMC 10372641. PMID 37459545. S2CID 259946428.
- ^ Nolan, M. R.; Walker, S. E.; Selly, T.; Schiffbauer, J. (2023). "Is the middle Cambrian Brooksella a hexactinellid sponge, trace fossil or pseudofossil?". PeerJ. 11. e14796. doi:10.7717/peerj.14796. PMC 9969855. PMID 36860767.
- ^ Wellman, C. H.; Lopes, G.; McKellar, Z.; Hartley, A. (2023). "Age of the basal 'Lower Old Red Sandstone' Stonehaven Group of Scotland: The oldest reported air-breathing land animal is Silurian (late Wenlock) in age". Journal of the Geological Society. 181. doi:10.1144/jgs2023-138. hdl:2164/22754.
- ^ Loron, C. C.; Rodriguez Dzul, E.; Orr, P. J.; Gromov, A. V.; Fraser, N. C.; McMahon, S. (2023). "Molecular fingerprints resolve affinities of Rhynie chert organic fossils". Nature Communications. 14 (1). 1387. Bibcode:2023NatCo..14.1387L. doi:10.1038/s41467-023-37047-1. PMC 10011563. PMID 36914650.
- ^ Sahoo, S. K.; Gilleaudeau, G. J.; Wilson, K.; Hart, B.; Barnes, B. D.; Faison, T.; Bowman, A. R.; Larsen, T. E.; Kaufman, A. J. (2023). "Basin-scale reconstruction of euxinia and Late Devonian mass extinctions". Nature. 615 (7953): 640–645. Bibcode:2023Natur.615..640S. doi:10.1038/s41586-023-05716-2. PMID 36890233. S2CID 257426134.
- ^ Shen, J.; Chen, J.; Yu, J.; Algeo, T. J.; Smith, R. M. H.; Botha, J.; Frank, T. D.; Fielding, C. R.; Ward, P. D.; Mather, T. A. (2023). "Mercury evidence from southern Pangea terrestrial sections for end-Permian global volcanic effects". Nature Communications. 14 (1). 6. Bibcode:2023NatCo..14....6S. doi:10.1038/s41467-022-35272-8. PMC 9810726. PMID 36596767.
- ^ Liu, F.; Peng, H.; Marshall, J. E. A.; Lomax, B. H.; Bomfleur, B.; Kent, M. S.; Fraser, W. T.; Jardine, P. E. (2023). "Dying in the Sun: Direct evidence for elevated UV-B radiation at the end-Permian mass extinction". Science Advances. 9 (1): eabo6102. Bibcode:2023SciA....9O6102L. doi:10.1126/sciadv.abo6102. PMC 9821938. PMID 36608140.
- ^ Seddon, A. W. R.; Zimmermann, B. (2023). "Comment on "Dying in the Sun: Direct evidence for elevated UV-B radiation at the end-Permian mass extinction"". Science Advances. 9 (34): eadi0570. Bibcode:2023SciA....9I.570S. doi:10.1126/sciadv.adi0570. PMC 10456835. PMID 37624886.
- ^ Jardine, P. E.; Peng, H.; Marshall, J. E. A.; Lomax, B. H.; Bomfleur, B.; Kent, M. S.; Fraser, W. T.; Liu, F. (2023). "Response to Comment on "Dying in the Sun: Direct evidence for elevated UV-B radiation at the end-Permian mass extinction"". Science Advances. 9 (34): eadj6309. Bibcode:2023SciA....9J6309J. doi:10.1126/sciadv.adj6309. PMC 10456830. PMID 37624883.
- ^ Lovelace, D. M.; Fitch, A. J.; Schwartz, D.; Schmitz, M. (2023). "Concurrence of Late Triassic lithostratigraphic, radioisotopic, and biostratigraphic data support a Carnian age for the Popo Agie Formation (Chugwater Group), Wyoming, USA". GSA Bulletin. doi:10.1130/B36807.1. S2CID 263809244.
- ^ Bond, A. D.; Dickson, A. J.; Ruhl, M.; Bos, R.; van de Schootbrugge, B. (2023). "Globally limited but severe shallow-shelf euxinia during the end-Triassic extinction". Nature Geoscience. 16 (12): 1181–1187. Bibcode:2023NatGe..16.1181B. doi:10.1038/s41561-023-01303-2.
- ^ Rodríguez-López, J. P.; Liesa, C. L.; Luzón, A.; Muñoz, A.; Mayayo, M. J.; Murton, J. B.; Soria, A. R. (2023). "Ice-rafted dropstones at midlatitudes in the Cretaceous of continental Iberia". Geology. 52: 33–38. doi:10.1130/G51725.1. S2CID 264094909.
- ^ Bryant, R.; Belanger, C. L. (2023). "Spatial heterogeneity in benthic foraminiferal assemblages tracks regional impacts of paleoenvironmental change across Cretaceous OAE2". Paleobiology. 49 (3): 431–453. Bibcode:2023Pbio...49..431B. doi:10.1017/pab.2022.47. S2CID 256132544.
- ^ Jones, M. M.; Sageman, B. B.; Selby, D.; Jacobson, A. D.; Batenburg, S. J.; Riquier, L.; MacLeod, K. G.; Huber, B. T.; Bogus, K. A.; Tejada, M. L. G.; Kuroda, J.; Hobbs, R. W. (2023). "Abrupt episode of mid-Cretaceous ocean acidification triggered by massive volcanism". Nature Geoscience. 16 (2): 169–174. Bibcode:2023NatGe..16..169J. doi:10.1038/s41561-022-01115-w. S2CID 256137367.
- ^ Callegaro, S.; Baker, D. R.; Renne, P. R.; Melluso, L.; Geraki, K.; Whitehouse, M. J.; De Min, A.; Marzoli, A. (2023). "Recurring volcanic winters during the latest Cretaceous: Sulfur and fluorine budgets of Deccan Traps lavas". Science Advances. 9 (40). eadg8284. Bibcode:2023SciA....9G8284C. doi:10.1126/sciadv.adg8284. PMC 10550224. PMID 37792933.
- ^ Siver, P. A.; Lott, A. M. (2023). "History of the Giraffe Pipe locality inferred from microfossil remains: a thriving freshwater ecosystem near the Arctic Circle during the warm Eocene". Journal of Paleontology. 97 (2): 271–291. Bibcode:2023JPal...97..271S. doi:10.1017/jpa.2022.101. S2CID 256435248.
- ^ Fidalgo, D.; Rosas, A.; Bartolini-Lucenti, S.; Boisserie, J.-R.; Pandolfi, L.; Martínez-Navarro, B.; Palmqvist, P.; Rook, L.; Madurell-Malapeira, J. (2023). "Increase on environmental seasonality through the European Early Pleistocene inferred from dental enamel hypoplasia". Scientific Reports. 13 (1). 16941. Bibcode:2023NatSR..1316941F. doi:10.1038/s41598-023-42936-y. PMC 10560273. PMID 37805524.
- ^ Abbas, M.; Lai, Z.; Jansen, J. D.; Tu, H.; Alqudah, M.; Xu, X.; Al-Saqarat, B. S.; Al Hseinat, M.; Ou, X.; Petraglia, M. D.; Carling, P. A. (2023). "Human dispersals out of Africa via the Levant". Science Advances. 9 (40). eadi6838. Bibcode:2023SciA....9I6838A. doi:10.1126/sciadv.adi6838. PMC 10550223. PMID 37792942.
- ^ Essel, E.; Zavala, E. I.; Schulz-Kornas, E.; Kozlikin, M. B.; Fewlass, H.; Vernot, B.; Shunkov, M. V.; Derevianko, A. P.; Douka, K.; Barnes, I.; Soulier, M.-C.; Schmidt, A.; Szymanski, M.; Tsanova, T.; Sirakov, N.; Endarova, E.; McPherron, S. P.; Hublin, J.-J.; Kelso, J.; Pääbo, S.; Hajdinjak, M.; Soressi, M.; Meyer, M. (2023). "Ancient human DNA recovered from a Palaeolithic pendant". Nature. 618 (7964): 328–332. Bibcode:2023Natur.618..328E. doi:10.1038/s41586-023-06035-2. PMC 10247382. PMID 37138083.
- ^ Reeves, J. C.; Sansom, R. S. (2023). "Multivariate mapping of ontogeny, taphonomy and phylogeny to reconstruct problematic fossil taxa". Proceedings of the Royal Society B: Biological Sciences. 290 (1999). 20230333. doi:10.1098/rspb.2023.0333. PMC 10229227. PMID 37253426.
- ^ Wang, W.; Shu, L.; Wang, D. (2023). "Soft body reconstruction of a reptile fossil by the nondestructive elemental mapping with a newly designed XRF". Island Arc. 32 (1). e12495. Bibcode:2023IsArc..32E2495W. doi:10.1111/iar.12495. S2CID 261057831.
- ^ Slater, T. S.; Ito, S.; Wakamatsu, K.; Zhang, F.; Sjövall, P.; Jarenmark, M.; Lindgren, J.; McNamara, M. E. (2023). "Taphonomic experiments reveal authentic molecular signals for fossil melanins and verify preservation of phaeomelanin in fossils". Nature Communications. 14 (1). 5651. Bibcode:2023NatCo..14.5651S. doi:10.1038/s41467-023-40570-w. PMC 10558522. PMID 37803012.
- ^ Peters, C.; Wang, Y.; Vakil, V.; Cramb, J.; Dortch, J.; Hocknull, S.; Lawrence, R.; Manne, T.; Monks, C.; Rössner, G. E.; Ryan, H.; Siversson, M.; Ziegler, T.; Louys, J.; Price, G. J.; Boivin, N.; Collins, M. J. (2023). "Bone collagen from subtropical Australia is preserved for more than 50,000 years". Communications Earth & Environment. 4 (1). 438. Bibcode:2023ComEE...4..438P. doi:10.1038/s43247-023-01114-8. hdl:10072/427854.
- ^ Brooke, C. F.; Marean, C. W.; Wren, C. D.; Bergin, S.; Fahey, B. P.; Venter, J. A. (2023). "Drivers of large mammal distribution: an overview and modelling approach for palaeoecological reconstructions of extinct ecosystems". Biological Journal of the Linnean Society. 141 (3): 307–322. doi:10.1093/biolinnean/blad100.
- ^ Hu, Y.; Li, X.; Boos, W. R.; Guo, J.; Lan, J.; Lin, Q.; Han, J.; Zhang, J.; Bao, X.; Yuan, S.; Wei, Q.; Liu, Y.; Yang, J.; Nie, J.; Guo, Z. (2023). "Emergence of the modern global monsoon from the Pangaea megamonsoon set by palaeogeography". Nature Geoscience. 16 (11): 1041–1046. Bibcode:2023NatGe..16.1041H. doi:10.1038/s41561-023-01288-y. S2CID 264363266.
- ^ Senel, C. B.; Kaskes, P.; Temel, O.; Vellekoop, J.; Goderis, S.; DePalma, R.; Prins, M. A.; Claeys, P.; Karatekin, Ö. (2023). "Chicxulub impact winter sustained by fine silicate dust". Nature Geoscience. 16 (11): 1033–1040. Bibcode:2023NatGe..16.1033S. doi:10.1038/s41561-023-01290-4.
- ^ The Cenozoic CO2 Proxy Integration Project (CenCO2PIP) Consortium (2023). "Toward a Cenozoic history of atmospheric CO2" (PDF). Science. 382 (6675). eadi5177. Bibcode:2023Sci...382i5177T. doi:10.1126/science.adi5177. PMID 38060645. S2CID 266054220.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Goto, K. T.; Tejada, M. L. G.; Tajika, E.; Suzuki, K. (2023). "Enhanced magmatism played a dominant role in triggering the Miocene Climatic Optimum". Communications Earth & Environment. 4 (1). 21. Bibcode:2023ComEE...4...21G. doi:10.1038/s43247-023-00684-x.
- ^ Wen, Y.; Zhang, L.; Holbourn, A. E.; Zhu, C.; Huntington, K. W.; Jin, T.; Li, Y.; Wang, C. (2023). "CO2-forced Late Miocene cooling and ecosystem reorganizations in East Asia". Proceedings of the National Academy of Sciences of the United States of America. 120 (5). e2214655120. Bibcode:2023PNAS..12014655W. doi:10.1073/pnas.2214655120. PMC 9945954. PMID 36689658. S2CID 256193615.
- ^ Lupien, R.; Uno, K.; Rose, C.; deRoberts, N.; Hazan, C.; de Menocal, P.; Polissar, P. (2023). "Low-frequency orbital variations controlled climatic and environmental cycles, amplitudes, and trends in northeast Africa during the Plio-Pleistocene". Communications Earth & Environment. 4 (1). 360. Bibcode:2023ComEE...4..360L. doi:10.1038/s43247-023-01034-7.
- ^ Margari, V.; Hodell, D. A.; Parfitt, S. A.; Ashton, N. M.; Grimalt, J. O.; Kim, H.; Yun, K.-S.; Gibbard, P. L.; Stringer, C. B.; Timmermann, A.; Tzedakis, P. C. (2023). "Extreme glacial cooling likely led to hominin depopulation of Europe in the Early Pleistocene". Science. 381 (6658): 693–699. Bibcode:2023Sci...381..693M. doi:10.1126/science.adf4445. hdl:10261/334363. PMID 37561880. S2CID 260776366.
