Voltaic pile
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (December 2010) |
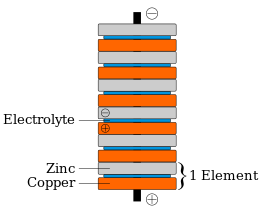


The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit.[1] It was invented by Italian chemist Alessandro Volta, who published his experiments in 1799.[2] Its invention can be traced back to an argument between Volta and Luigi Galvani, Volta's fellow Italian scientist who had conducted experiments on frogs' legs.[3] Use of the voltaic pile enabled a rapid series of other discoveries, including the electrical decomposition (electrolysis) of water into oxygen and hydrogen by William Nicholson and Anthony Carlisle (1800), and the discovery or isolation of the chemical elements sodium (1807), potassium (1807), calcium (1808), boron (1808), barium (1808), strontium (1808), and magnesium (1808) by Humphry Davy.[4][5]
The entire 19th-century electrical industry was powered by batteries related to Volta's (e.g. the Daniell cell and Grove cell) until the advent of the dynamo (the electrical generator) in the 1870s.[6]
History
[edit]Volta's invention was built on Luigi Galvani's 1780s discovery that a circuit of two metals and a frog's leg can cause the frog's leg to respond.[1] Volta demonstrated in 1794 that when two metals and brine-soaked cloth or cardboard are arranged in a circuit they too produce an electric current. In 1800, Volta stacked several pairs of alternating copper (or silver) and zinc discs (electrodes) separated by cloth or cardboard soaked in brine, which increased the total electromotive force.[7][8] When the top and bottom contacts were connected by a wire, an electric current flowed through the voltaic pile and the connecting wire. This was the first "true" battery, that gave off continuous charge.[9]
Many scientific instruments that belonged to Alessandro Volta are preserved in the University History Museum of the University of Pavia, where Volta taught from 1778 to 1819; the piles on display, unfortunately, are not original, as the ones preserved in Pavia were lent on the occasion of the centenary of the invention and subsequently lost in a fire.[10]
Applications
[edit]
On 20 March 1800, Alessandro Volta wrote to the London Royal Society to describe the technique for producing electric current using his device.[11] On learning of the voltaic pile, William Nicholson and Anthony Carlisle used it to discover the electrolysis of water. Humphry Davy showed that the electromotive force, which drives the electric current through a circuit containing a single voltaic cell, was caused by a chemical reaction, not by the voltage difference between the two metals. He also used the voltaic pile to decompose chemicals and to produce new chemicals. William Hyde Wollaston showed that electricity from voltaic piles had identical effects to those of electricity produced by friction. In 1802 Vasily Petrov used voltaic piles in the discovery and research of electric arc effects.
Humphry Davy and Andrew Crosse were among the first to develop large voltaic piles.[12] Davy used a 2000-pair pile made for the Royal Institution in 1808 to demonstrate carbon arc discharge[13] and isolate five new elements: barium, calcium, boron, strontium and magnesium.[14]
Electrochemistry
[edit]Because Volta believed that the electromotive force occurred at the contact between the two metals, Volta's piles had a different design than the modern design illustrated on this page. His piles had one extra disc of copper at the top, in contact with the zinc, and one extra disc of zinc at the bottom, in contact with the copper.[15] Expanding on Volta's work and the electro-magnetism work of his mentor Humphry Davy, Michael Faraday utilized both magnets and the voltaic pile in his experiments with electricity. Faraday believed that all "electricities" being studied at the time (voltaic, magnetic, thermal, and animal) were one and the same. His work to prove this theory led him to propose two laws of electrochemistry which stood in direct conflict with the current scientific beliefs of the day as laid down by Volta thirty years earlier.[16] Because of their contributions to the understanding of this field of study, Faraday and Volta are both considered to be among the fathers of electrochemistry.[17] The words "electrode" and "electrolyte", used above to describe Volta's work, are due to Faraday.[18]
Electromotive force
[edit]The strength of the pile is expressed in terms of its electromotive force, or emf, given in volts. Alessandro Volta's theory of contact tension considered that the emf, which drives the electric current through a circuit containing a voltaic cell, occurs at the contact between the two metals. Volta did not consider the electrolyte, which was typically brine in his experiments, to be significant. However, chemists soon realized that water in the electrolyte was involved in the pile's chemical reactions, and led to the evolution of hydrogen gas from the copper or silver electrode.[4][19][20][21]
The modern, atomistic understanding of a cell with zinc and copper electrodes separated by an electrolyte is the following. When the cell is providing an electrical current through an external circuit, the metallic zinc at the surface of the zinc anode is oxidized and dissolves into the electrolyte as electrically charged ions (Zn2+), leaving two negatively charged electrons (
e−
) behind in the metal:
- anode (oxidation): Zn
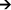 Zn2+ + 2
Zn2+ + 2
e−
- anode (oxidation): Zn
This reaction is called oxidation. While zinc is entering the electrolyte, two positively charged hydrogen ions (H+) from the electrolyte accept two electrons at the copper cathode surface, become reduced and form an uncharged hydrogen molecule (H2):
- cathode (reduction): 2 H+ + 2
e−
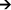 H2
H2
- cathode (reduction): 2 H+ + 2
This reaction is called reduction. The electrons used from the copper to form the molecules of hydrogen are made up by an external wire or circuit that connects it to the zinc. The hydrogen molecules formed on the surface of the copper by the reduction reaction ultimately bubble away as hydrogen gas.
One will observe that the global electro-chemical reaction does not immediately involve the electrochemical couple Cu2+/Cu (Ox/Red) corresponding to the copper cathode. The copper metal disk thus only serves here as a "chemically inert" noble metallic conductor for the transport of electrons in the circuit and does not chemically participate in the reaction in the aqueous phase. Copper does act as a catalyst for the hydrogen-evolution reaction, which otherwise could occur equally well directly at the zinc electrode without current flow through the external circuit. The copper electrode could be replaced in the system by any sufficiently noble/inert and catalytically active metallic conductor (Ag, Pt, stainless steel, graphite, ...). The global reaction can be written as follows:
- Zn + 2H+
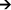 Zn2+ + H2
Zn2+ + H2
- Zn + 2H+
This is usefully stylized by means of the electro-chemical chain notation:
- (anode: oxidation) Zn | Zn2+ || 2H+ | H2 | Cu (cathode: reduction)
in which a vertical bar each time represents an interface. The double vertical bar represents the interfaces corresponding to the electrolyte impregnating the porous cardboard disk.
When no current is drawn from the pile, each cell, consisting of zinc/electrolyte/copper, generates 0.76 V with a brine electrolyte. The voltages from the cells in the pile add, so the six cells in the diagram above generate 4.56 V of electromotive force.
Dry piles
[edit]A number of high-voltage dry piles were invented between 1800 and the 1830s in an attempt to determine the source of electricity of the wet voltaic pile, and specifically to support Volta's hypothesis of contact tension. Indeed, Volta himself experimented with a pile whose cardboard discs had dried out, most likely accidentally.
The first to publish the discovery of a dry pile that produced a current was Johann Wilhelm Ritter in 1802, albeit in an obscure journal; over the next decade, it was announced repeatedly as a new discovery. One form of dry pile is the Zamboni pile. Francis Ronalds in 1814 was one of the first to realize that dry piles also worked through chemical reaction rather than metal-to-metal contact, even though corrosion was not visible due to the very small currents generated.[22][23]
The dry pile could be referred to as the ancestor of the modern dry cell.[original research?]
See also
[edit]References
[edit]- ^ a b "Battery: Voltaic Pile". americanhistory.si.edu. Retrieved 2024-05-12.
- ^ "Alessandro Volta | Biography, Facts, Battery, & Invention | Britannica". www.britannica.com. 2024-04-15. Retrieved 2024-05-12.
- ^ "The Voltaic Pile | Distinctive Collections Spotlights". libraries.mit.edu. Retrieved 2023-01-24.
- ^ a b Decker, Franco (January 2005). "Volta and the 'Pile'". Electrochemistry Encyclopedia. Case Western Reserve University. Archived from the original on 2012-07-16.
- ^ Russell, Colin (August 2003). "Enterprise and electrolysis..." Chemistry World.
- ^ "Alessandro Volta | Biography, Facts, Battery, & Invention | Britannica". www.britannica.com. 2024-04-15. Retrieved 2024-05-12.
- ^ Brockman, C. J. (June 1927). "Primary cells—A brief historical sketch". Journal of Chemical Education. 4 (6): 770. doi:10.1021/ed004p770. ISSN 0021-9584.
- ^ Mottelay, Paul Fleury (2008). Bibliographical History of Electricity and Magnetism (Reprint of 1892 ed.). Read Books. p. 247. ISBN 978-1-4437-2844-7.
- ^ "The Voltaic Pile | Distinctive Collections Spotlights". libraries.mit.edu. Retrieved 2023-01-24.
- ^ "Sala Volta". Musei Unipv. Retrieved 21 August 2022.
- ^ Volta, Alessandro (1800). "On the Electricity Excited by the Mere Contact of Conducting Substances of Different Kinds". Philosophical Transactions of the Royal Society of London (in French). 90: 403–431. doi:10.1098/rstl.1800.0018. A partial translation of this paper is available online; see "Volta and the Battery". Retrieved 2012-12-01. A complete translation was published in Dibner, Bern (1964). Alessandro Volta and the Electric Battery. Franklin Watts. pp. 111–131. OCLC 247967.
- ^ Encyclopædia Britannica, 1911 edition, Volume V09, Page 185
- ^ Tracking Down the Origin of Arc Plasma Science. II. Early Continuous Discharges
- ^ Kenyon, T. K. (2008). "Science and Celebrity: Humphry Davy's Rising Star". Chemical Heritage Magazine. 26 (4): 30–35. Retrieved 22 March 2018.
- ^ Cecchini, R.; Pelosi, G. (April 1992). "Alessandro Volta and his battery". IEEE Antennas and Propagation Magazine. 34 (2): 30–37. Bibcode:1992IAPM...34...30C. doi:10.1109/74.134307. S2CID 6515671.
- ^ James, Frank A. J. L. (1989). "Michael Faraday's first law of electrochemistry: how context develops new knowledge". In Stock, J. T.; Orna, M. V. (eds.). Electrochemistry, past and present. Washington, DC: American Chemical Society. pp. 32–49. ISBN 9780841215726.
- ^ Stock, John T. (1989). "Electrochemistry in retrospect: an overview". In Orna, Mary Virginia (ed.). Electrochemistry, past and present. Washington, DC: American Chemical Society. pp. 1–17. ISBN 9780841215726.
- ^ James, F.A.J.L. (18 July 2013). "The Royal Institution of Great Britain: 200 years of scientific discovery and communication". Interdisciplinary Science Reviews. 24 (3): 225–231. doi:10.1179/030801899678777.
- ^ Turner, Edward (1841). Liebig, Justus; Gregory, William (eds.). Elements of chemistry: including the actual state and prevalent doctrines of the science (7 ed.). London: Taylor and Walton. p. 102.
During the action of a simple circle, as of zinc and copper, excited by dilute sulfuric acid, all of the hydrogen developed in the voltaic action is evolved at the surface of the copper.
- ^ Goodisman, Jerry (2001). "Observations on Lemon Cells". Journal of Chemical Education. 78 (4): 516. Bibcode:2001JChEd..78..516G. doi:10.1021/ed078p516. Goodisman notes that many chemistry textbooks use an incorrect model for a cell with zinc and copper electrodes in an acidic electrolyte.
- ^ Graham-Cumming, John (2009). "Tempio Voltiano". The Geek Atlas: 128 Places Where Science and Technology Come Alive. O'Reilly Media. p. 97. ISBN 9780596523206.
- ^ Ronalds, B.F. (2016). Sir Francis Ronalds: Father of the Electric Telegraph. London: Imperial College Press. ISBN 978-1-78326-917-4.
- ^ Ronalds, B.F. (July 2016). "Francis Ronalds (1788-1873): The First Electrical Engineer?". Proceedings of the IEEE. 104 (7): 1489–1498. doi:10.1109/JPROC.2016.2571358. S2CID 20662894.
External links
[edit]- "Voltaic Pile Tutorial". National High Magnetic Field Laboratory.
- "The Voltaic Pile". Electricity. Kenyon.edu.
- Lewis, Nancy D., "Alesandro Volta The Voltaic Pile".
- Lewis, Nancy D., "Humphry Davy Electrochemistry".

