Lithium hybrid organic battery
Lithium hybrid organic batteries are an energy storage device that combines lithium with an organic polymer. For example, polyaniline vanadium (V) oxide (PAni/V2O5) can be incorporated into the nitroxide-polymer lithium iron phosphate battery, PTMA/LiFePO4. Together, they improve the lithium ion intercalation capacity, cycle life, electrochemical performances, and conductivity of batteries.
PAni/V2O5
[edit]Oxides, like V2O5, are used as cathodes in rechargeable lithium batteries. Crystalline V2O5 has a weaker rechargeability or cyclability than amorphous V2O5 because the crystal structure is damaged during discharge/charge cycles.[1] However, amorphous oxides, in particular the V2O5 xerogel, allows lithium ions to diffuse faster and thus have a better cyclability. Hybrid is formed by combining a conducting organic polymer (e.g. polyaniline) with an oxide (e.g. V2O5).
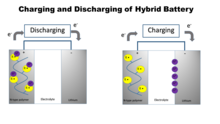


V2O5 gels are prepared using the ion-exchange method.[2] Vanadium (V) polymerizes aniline. Before synthesis of a hybrid battery, potentiometric titration of V2O5 gel with is carried out; this determines the amount of V(V) present in the gel. Aniline solution is slowly added onto the gel. The following procedure is demonstrated in Figure 3.
V2O5 is used because of its high specific capacity, high thermal stability, and high structural flexibility with lithium.[3] Up to three moles of lithium ions can be added into the V2O5 lattice to create different structures.[4] The structures created give the hybrid a long battery life. However, the intercalation capacity depends on the moderate electrical conductivity and low diffusion coefficient of the lithium ions in the vanadium oxide matrix.[5]
Polyaniline is easily produced to have controlled structural and electronic properties.[6] Polyaniline eliminates the coordinated water of the V2O5 xerogel, so more lithium ions can be integrated into the structure. The organic part of the PAni/V2O5 hybrid degrades with the increase of temperature.[7]
V(V) is reduced to V(IV), and aniline is oxidized to polyaniline.[8] Re-oxidizing V(IV) to a higher oxidation state of V(V) increases initial cell voltage and specific capacity. Since polyaniline is an electrochemically active component, it improves the specific charge of the hybrid material.[citation needed] Combining polyaniline with V2O5 yields a larger specific charge difference. Thus, a greater total capacity contribution than V2O5 alone. Furthermore, the hybrid has a higher specific capacity than that of the V2O5 xerogel. Electrical conductivity is as high as 0.09 S/cm for 15 days.[9]
As a result, PAni/V2O5 hybrid is a conducting network and an electroactive material in the composites, which improves electrochemical behavior. It also prevents the irreversible structural changes made by redox cycling when the lithium ions enter the lattice. Moreover, this hybrid also has a high specific capacity and improved cyclability without capacity deterioration.
PTMA/LiFePO4
[edit]PTMA is an organic nitroxide radical electrode-active polymer, and LiFePO4 is the inorganic electrode-active material. PTMA is used because it has a high capacity and a long cycle life.[10] To synthesize organic radical-inorganic hybrid electrodes, electrode environments for each component must be optimized. PTMA and LiFePO4 were combined with entire PTMA and LiFePO4 electrode with different weight ratios: 25/75, 50/50, and 75/25.[11]

The cell was prepared by using a working electrode to assemble a half-cell configuration dry glove box with Li metal as an anode, ethyl carbonate/dimethyl carbonate as an electrophile, and a Celgard 3501 membrane as a separator. Using Arbin BT-200 Battery Tester, the cell was electrochemically cycled at room temperature. By using a Solarton workstation, the cyclic voltammetry and electrochemical impedance spectroscopy of cells were performed. A focus ion beam-scanning electron microscope was used to determine the morphology of the electrodes before and after the high rate pulse discharge (HRPD) cycling.[12]
After testing, pure PTMA and LiFePO4 electrode give a sharp redox peak and decrease the voltage gap between oxidation and reduction.[13] Therefore, PTMA and LiFePO4 improve the rate and reversibility of the redox couples. Furthermore, the hybrid cathodes have a lower charge-transfer resistance, allowing easier migration of Li ions through the electrode interface. Moreover, PTMA/LiFePO4 has a longer life cycle compared to pure LiFePO4 or PTMA systems.
References
[edit]- ^ Ng, S. H., Chew, S. Y., Wang, J., Wexler, D., Tournayre, Y., Konstantinov, K., & Liu, H. K. (2007). Synthesis and electrochemical properties of V 2 O 5 nanostructures prepared via a precipitation process for lithium-ion battery cathodes. Journal of Power Sources, 174(2), 1032-1035.
- ^ Liu, D., Liu, Y., Garcia, B. B., Zhang, Q., Pan, A., Jeong, Y. H., & Cao, G. (2009). V2O5 xerogel electrodes with much enhanced lithium-ion intercalation properties with N 2 annealing. Journal of Materials Chemistry, 19(46), 8789-8795. doi:10.1039/b914436f.
- ^ Chao, D., Xia, X., Liu, J., Fan, Z., Ng, C. F., Lin, J., ... & Fan, H. J. (2014). A V2O5/Conductive‐Polymer Core/Shell Nanobelt Array on Three‐Dimensional Graphite Foam: A High‐Rate, Ultrastable, and Freestanding Cathode for Lithium‐Ion Batteries. Advanced Materials, 26(33), 5794-5800. doi:10.1002/adma.201400719.
- ^ Swider-Lyons, K. E., Love, C. T., & Rolison, D. R. (2002). Improved lithium capacity of defective V2O5 materials. Solid State Ionics, 152, 99-104. doi:10.1016/S0167-2738(02)00350-8.
- ^ Li, G., Lu, Z., Huang, B., Huang, H., Xue, R., & Chen, L. (1995). An evaluation of lithium intercalation capacity into carbon by XRD parameters. Solid state ionics, 81(1), 15-18. doi:10.1016/0167-2738(95)00166-4.
- ^ Molapo, K. M., Ndangili, P. M., Ajayi, R. F., Mbambisa, G., Mailu, S. M., Njomo, N., ... & Iwuoha, E. I. (2012). Electronics of conjugated polymers (I): polyaniline.International Journal of Electrochemical Science, 7(12).
- ^ Lira-Cantu, M., Gomez-Romero, P. (1999), The Organic-Inorganic Polyaniline/V2O5 System: Application as a High Capacity Hybrid Cathode for Rechargeable Lithium Batteries. Journal of the Electrochemical Society, 146(6): 2029-2033. doi:10.1149/1.1391886.
- ^ Lira-Cantu, M., Gomez-Romero, P. (1999), The Organic-Inorganic Polyaniline/V2O5 System: Application as a High Capacity Hybrid Cathode for Rechargeable Lithium Batteries. Journal of the Electrochemical Society, 146(6): 2029-2033. doi:10.1149/1.1391886.
- ^ Park, K., Song, H., Kim, Y., Mho, S., Cho, W., and Yeo. I. (2009), Electrochemical Preparation and Characterization of V2O5 / Polyaniline composite Film Cathodes for Li Battery. Electrochimica Acta: Emerging Trends and Challenges in Electrochemistry, 55(27): 8023-8029. doi:10.1016/j.electacta.2009.12.047.
- ^ Guo, W., Yin, Y., Xin, S., Guo Y., and Wan, L. (2011), Superior Radical Polymer Cathode Material with a Two-electron Process Redox Reaction Promoted by Graphene. Energy and Environmental Science, 5(1): 5221-5225. doi: 10.1039/c1ee02148f.
- ^ Huang, Q., Cosimbescu, L., Koech, P., Choi, D., and Lemmon, J. (2013), Composite Organic Radical-Inorganic Hybrid Cathode for Lithium-Ion Batteries. Journal of Power Sources, 233(3): 69-73. doi:10.1016/j.jpowsour.2013.01.076.
- ^ Huang, Q., Cosimbescu, L., Koech, P., Choi, D., and Lemmon, J. (2013), Composite Organic Radical-Inorganic Hybrid Cathode for Lithium-Ion Batteries. Journal of Power Sources, 233(3): 69-73. doi:10.1016/j.jpowsour.2013.01.076.
- ^ Vlad, A., Singh, N., Rolland, J., Melinte, S., Ajayan, P. M., & Gohy, J. F. (2014). Hybrid supercapacitor-battery materials for fast electrochemical charge storage.Scientific reports, 4. doi:10.1038/sren04315.


