User:SettleGod/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Epidiolex |
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 13–19% (oral),[1] 11–45% (mean 31%; inhaled)[2] |
| Elimination half-life | 9 h[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.4636 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 66 °C (151 °F) |
| Boiling point | 180 °C (356 °F) (range: 160–180 °C)[3] |
| |
| |
| (verify) | |
| Part of a series on |
| Cannabis |
|---|
 |
Cannabidiol (CBD) is one of at least 113 active cannabinoids identified in cannabis.[4][5] It is a major phytocannabinoid, accounting for up to 40% of the plant's extract.[6] CBD is considered to have a wide scope of potential medical applications despite its reputed lack of psychoactivity.
Research
[edit]
Neurological effects
[edit]Cannabidiol has been seen to be an anticonvulsant in animals, but controlled studies in humans are lacking.[7]
Transdermal CBD is neuroprotective in animals.[8]
Dravet syndrome
[edit]Dravet syndrome is a rare form of epilepsy that is difficult to treat. It is a catastrophic form of intractable epilepsy that begins in infancy. Initial seizures are most often prolonged events and in the second year of life other seizure types begin to emerge.[9] A number of high profile and anecdotal reports have sparked interest in treatment of Dravet syndrome with CBD.[10] GW Pharmaceuticals is seeking FDA approval to market a formulation of CBD, under the tradename Epidiolex, as a treatment for Dravet syndrome. Epidiolex was granted fast-track status and is in late stage trials following positive early results from the drug.[10][11][12][13][14] Some cannabis extract preparations containing CBD are marketed as dietary supplements and claim efficacy against Dravet Syndrome. One such preparation is marketed under the tradename Charlotte's web.[15][16]
Psychotropic effect
[edit]A 2014 Cochrane Review concluded that the evidence is insufficient to conclude that CBD has anti-psychotic effects.[17] Others have concluded it may have antipsychotic effects and may counteract the potential psychotomimetic effects of THC on individuals with latent schizophrenia;[18] some reports show it to be an alternative treatment for schizophrenia that is safe and well-tolerated.[19] Studies have shown CBD may reduce schizophrenic symptoms due to its apparent ability to stabilize disrupted or disabled NMDA receptor pathways in the brain, which are shared and sometimes contested by norepinephrine and GABA.[19][20] Studies have shown cannabidiol decreases activity of the limbic system [21] and decreases social isolation induced by THC in rats.[22] Additionally, a study focusing on the inhibition of cocaine-induced seizures in rats displayed the potential anti-epileptic benefits of CBD.[23]
Chronic cannabidiol administration in rats was found to produce reactions suggesting anxiety, indicating that prolonged treatment with cannabidiol might lead to anxiety.[24] Those results have been contested by Gururajan,[25] and contradict Réus,[26] whose experimentation cover the same duration. The results of one study indicate that the administration of cannabidiol may reduce subjective anxiety in patients diagnosed with SAD.[27]
Research on cannabidiol has shown its efficacy in improving the psychotic symptoms of patients with Schizophrenia by elevating levels of anandamide, an antipsychotic endocannabinoid, in the body. These studies also showed that patients given CBD displayed less adverse side-effects than patients given other antipsychotic drugs.[28] Patients with Huntington's disease reported reduction in chorea severity after taking CBD.[29]
Other research indicates CBD as a possible form of therapy and intervention for those suffering from substance abuse, due to its association with the neural circuits involved in addictive behaviors. CBD has an effect on the 5-HT1A receptor which controls stress responses and compulsive behaviors.[30] Studies in rats show that CBD has in impact on opioid addiction and psychostimulant addiction. There have been studies showing cannabidiol as having a positive impact on cannabis and tobacco addictions in humans.[30]
CBD-enhanced cannabis
[edit]Selective breeding by growers in the USA dramatically lowered the CBD content of cannabis; their customers preferred varietals that were more mind-altering due to a higher THC, lower CBD content.[31] This is because cannabidiol's relatively low affinity for the CB1 and CB2 receptors does not produce the effects of intoxication and memory loss commonly experienced by THC users.[32] These individuals are generally less interested in alleviating medical symptoms, and more interested in pursuing the euphoria-inducing effects of cannabis with a higher THC, lower CBD content. However, it is significant to consider the synergistic nature of the two cannabinoids and their ability to enhance one another's therapeutic effects.[33] For example, research suggests CBD may augment the anti-inflammatory benefits of THC. The two molecules in combination have also been reported to more aptly reduce neuropathic pain in users.[33] To meet the demands of medical cannabis patients, growers are currently developing more CBD-rich strains.[34]
Industrial hemp
[edit]Several industrial hemp varieties can be legally cultivated in western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1% cannabidiol (CBD) with THC less than 0.1%.[35]
Extraction can be done with olive oil, ethanol, or CO2, and other nonpolar to semipolar solvents.[36]
Pharmacodynamics
[edit]Cannabidiol has a very low affinity for CB1 and CB2 receptors but acts as an indirect antagonist of their agonists.[37][38] While one would assume that this would cause cannabidiol to reduce the effects of THC, it may potentiate THC's effects by increasing CB1 receptor density or through another CB1-related mechanism.[39] It may also extend the duration of the effects of THC via inhibition of the cytochrome P-450-3A and 2C enzymes.[40]
Recently, it was found to be an antagonist at the putative new cannabinoid receptor, GPR55, a GPCR expressed in the caudate nucleus and putamen.[41] Cannabidiol has also been shown to act as a 5-HT1A receptor partial agonist,[42] an action which may be involved in its antidepressant,[43][44] anxiolytic,[44][45] and neuroprotective[46][47] effects. Cannabidiol is an allosteric modulator of μ and δ-opioid receptors.[48] Cannabidiol's pharmacological effects have also been attributed to PPAR-γ receptor agonism and intracellular calcium release.[18]
Research suggests that CBD may exert some of its pharmacological action through its inhibition of FAAH, which may in turn increase the levels of endocannabinoids, such as anandamide, produced by the body.[6] Cannabidiol may act as an agonist of VR1, desensitizing it to the action of capsaicin, which partially potentiates CBD as an anti-inflammatory drug.[49]
Pharmacokinetic interactions
[edit]There is some preclinical evidence to suggest that cannabidiol may reduce THC clearance, modestly increasing THC's plasma concentrations resulting in a greater amount of THC available to receptors, increasing the effect of THC in a dose-dependent manner.[50][51] Despite this the available evidence in humans suggests no significant effect of CBD on THC plasma levels.[52]
Pharmaceutical preparations
[edit]Nabiximols (USAN, trade name Sativex) is an aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC. The drug was approved by Canadian authorities in 2005 to alleviate pain associated with multiple sclerosis.[53][54][55]
Epidiolex is an oil formulation of CBD extracted from the cannabis plant undergoing clinical trials for refractory epilepsy syndromes.[56]
Isomerism
[edit]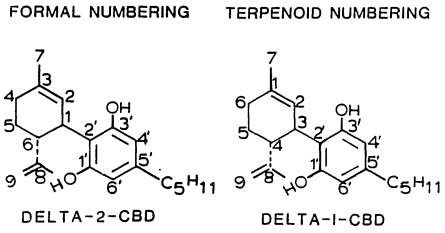
| 7 double bond isomers and their 30 stereoisomers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Formal numbering | Terpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ5-cannabidiol | 1 and 3 | 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ4-cannabidiol | 1 and 3 | 4 | No | unscheduled | 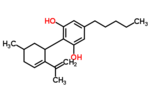
|
| Δ4-cannabidiol | 1, 3 and 6 | 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ5-cannabidiol | 1, 3 and 4 | 8 | No | unscheduled | 
|
| Δ3-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ6-cannabidiol | 3 and 4 | 4 | ? | unscheduled | 
|
| Δ3,7-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol | Δ1,7-cannabidiol | 3 and 4 | 4 | No | unscheduled | 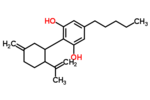
|
| Δ2-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ1-cannabidiol | 3 and 4 | 4 | Yes | unscheduled | 
|
| Δ1-cannabidiol | 3 and 6 | 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ2-cannabidiol | 1 and 4 | 4 | No | unscheduled | 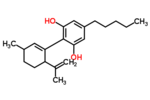
|
| Δ6-cannabidiol | 3 | 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ3-cannabidiol | 1 | 2 | No | unscheduled | 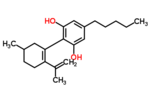
|
See also: Tetrahydrocannabinol#Isomerism, Abnormal cannabidiol.
Chemistry
[edit]Cannabidiol is insoluble in water but soluble in organic solvents such as pentane. At room temperature, it is a colorless crystalline solid.[57] In strongly basic media and the presence of air, it is oxidized to a quinone.[58] Under acidic conditions it cyclizes to THC.[59] The synthesis of cannabidiol has been accomplished by several research groups, commonly through the combination of Olivetol and p-mentha-2,8-dien-1-ol in the presence of Boron trifluoride.[60][61][62]
Biosynthesis
[edit]Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC. An acid-catalyzed reaction between geranylpyrophosphate and olivetolate produces Cannabigerolic acid. In the last step, however, CBD acid synthase is used to yield CBDA rather than THC acid synthase being used to produce THCA.[36][63]

Legal status
[edit]Cannabidiol is not scheduled by the Convention on Psychotropic Substances.
Australia
[edit]Prescription Medicine (Schedule 4).[64]
Canada
[edit]Cannabidiol is a Schedule II drug in Canada.[65]
United States
[edit]Epidiolex, a cannabidiol-containing medication, has received orphan drug status in the United States for treatment of Dravet syndrome, which will allow it to be studied.[66] Though the extraction of CBD from industrial hemp is currently legally permitted throughout the United States, extracting CBD from medical marijuana plants may only be performed in states in which medical marijuana is legal.[33]
Legislation has been passed in several states which allows for the medicinal use of products high in CBD. One piece of such legislation is Carly's Law, passed in Alabama in April of 2014, which permits the prescribed use of cannabidiol for patients with severely-debilitating epilepsy.[67] In Alabama, CBD extracts may not contain plant material and must contain < 3% of THC.[68]
UK
[edit]Cannabidiol, in an oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, is a prescription product available for relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective).[69]
EU
[edit]Cannabidiol is listed in EU Cosmetics Ingredient Database.[70]
References
[edit]- ^ a b Mechoulam R, Parker LA, Gallily R (November 2002). "Cannabidiol: an overview of some pharmacological aspects". The Journal of Clinical Pharmacology (Review). 42 (11 Suppl): 11S–19S. doi:10.1002/j.1552-4604.2002.tb05998.x. PMID 12412831. S2CID 10024088.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytotherapy Research (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286. S2CID 21836765.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ [unreliable medical source?] McPartland JM, Russo EB (2001). "Cannabis and cannabis extracts: greater than the sum of their parts?" (PDF). Journal of Cannabis Therapeutics. 1 (3/4): 103–132. doi:10.1300/J175v01n03_08.
- ^ Borgelt, Laura M.; Franson, Kari L.; Nussbaum, Abraham M.; Wang, George S. (5 February 2013). "The Pharmacologic and Clinical Effects of Medical Cannabis". Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 33 (2): 195–209. doi:10.1002/phar.1187. PMID 23386598. S2CID 8503107.
- ^ Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2 February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ a b Campos, A. C.; Moreira, F. A.; Gomes, F. V.; Del Bel, E. A.; Guimaraes, F. S. (2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society B: Biological Sciences. 367 (1607): 3364–3378. doi:10.1098/rstb.2011.0389. ISSN 0962-8436. PMC 3481531. PMID 23108553.
- ^ Devinsky, Orrin; Cilio, Maria Roberta; Cross, Helen; Fernandez-Ruiz, Javier; French, Jacqueline; Hill, Charlotte; Katz, Russell; Di Marzo, Vincenzo; Jutras-Aswad, Didier (2014-06-01). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631. ISSN 1528-1167. PMC 4707667. PMID 24854329.
- ^ Liput DJ, Hammell DC, Stinchcomb AL, Nixon K (2013). "Transdermal delivery of cannabidiol attenuates binge alcohol-induced neurodegeneration in a rodent model of an alcohol use disorder". Pharmacology Biochemistry and Behavior. 111: 120–7. doi:10.1016/j.pbb.2013.08.013. PMC 4096899. PMID 24012796.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.dravetfoundation.org/dravet-syndrome/what-is-dravet-syndrome#sthash.jAC0bZ89.dpuf What is Dravet Syndrome?
- ^ a b Melville, Nancy A. (14 Aug 2013), Seizure Disorders Enter Medical Marijuana Debate, Medscape Medical News., retrieved 2014-01-14
{{citation}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Throckmorton, Douglas (24 June 2015). "Cannabidiol: Barriers to Research and Potential Medical Benefits". FDA. FDA. Retrieved 15 December 2015.
- ^ Gloss D, Vickrey B (13 June 2012). Gloss, David (ed.). "Cannabinoids for epilepsy". The Cochrane Database of Systematic Reviews (Review). 6 (6): CD009270. doi:10.1002/14651858.CD009270.pub2. PMID 22696383.
- ^ Devinsky, Orrin (2015). "Efficacy and Safety of Epidiolex (Cannabidiol) in Children and Young Adults with Treatment-Resistant Epilepsy". Annual Meeting Abstracts. American Epilepsy Society. Retrieved 13 December 2015.
- ^ Angus, Chen (8 December 2015). "Marijuana's Main Ingredient, Cannabidiol, May Be An Effective Way To Treat Epilepsy". Medical Daily. Retrieved 14 December 2015.
- ^ Maa, Edward; Figi, Paige (2014). "The case for medical marijuana in epilepsy". Epilepsia. 55 (6): 783–786. doi:10.1111/epi.12610. ISSN 0013-9580. PMID 24854149. S2CID 4849160.
- ^ Young, Saundra. "Marijuana stops child's severe seizures" (PDF). CNN. CNN. Retrieved 7 January 2016.
- ^ McLoughlin BC, Pushpa-Rajah JA, Gillies D, Rathbone J, Variend H, Kalakouti E, Kyprianou K (2014). "Cannabis and schizophrenia". The Cochrane Database of Systematic Reviews. 10 (10): CD004837. doi:10.1002/14651858.CD004837.pub3. PMID 25314586. S2CID 8434704.
- ^ a b Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 367 (1607): 3364–78. doi:10.1098/rstb.2011.0389. PMC 3481531. PMID 23108553.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimarães FS (April 2006). "Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug". Brazilian Journal of Medical and Biological Research (Review). 39 (4): 421–9. doi:10.1590/S0100-879X2006000400001. PMID 16612464.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Long LE, Malone DT, Taylor DA (2005). "Cannabidiol Reverses MK-801-Induced Disruption of Prepulse Inhibition in Mice". Neuropsychopharmacology. 31 (4): 795–803. doi:10.1038/sj.npp.1300838. PMID 16052245. S2CID 14611952.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JE, McGuire PK, Filho Busatto G (October 2003). "Effects of Cannabidiol (CBD) on Regional Cerebral Blood Flow". Neuropsychopharmacology. 29 (2): 417–426. doi:10.1038/sj.npp.1300340. PMID 14583744. S2CID 10877499.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Malone DT, Jongejan D, Taylor DA (August 2009). "Cannabidiol reverses the reduction in social interaction produced by low dose Δ9-tetrahydrocannabinol in rats". Pharmacology Biochemistry and Behavior. 93 (2): 91–96. doi:10.1016/j.pbb.2009.04.010. PMID 19393686. S2CID 8187195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gobiraa, P.H., Vilelaa, L.R., Gonçalvesa, B., Santosa, R.P.M., deOliveiraa, A., Vieiraa, L.B., Aguiara, D.C., Crippab, J.A. (September 2015). "Cannabidiol, a Cannabis sativa constituent, inhibits cocaine-induced seizures in mice: Possible role of the mTOR pathway and reduction in glutamate release". NeuroToxicology. 50: 116–121. doi:10.1016/j.neuro.2015.08.007. PMID 26283212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ ElBatsh MM, Assareh N, Marsden CA, Kendall DA (May 2012). "Anxiogenic-like effects of chronic cannabidiol administration in rats". Psychopharmacology. 221 (2): 239–247. doi:10.1007/s00213-011-2566-z. PMID 22083592. S2CID 8387741.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gururajan A (2012). "Comment on: "Anxiogenic-like effects of chronic cannabidiol administration in rats" (Elbatsh MM, Assareh N, Marsden CA, Kendall DA, Psychopharmacology 2012)". Psychopharmacology. 222 (4): 725–6, author reply 727. doi:10.1007/s00213-012-2780-3. PMID 22760485. S2CID 10920194.
- ^ Réus GZ, Stringari RB, Ribeiro KF, Luft T, Abelaira HM, Fries GR, Aguiar BW, Kapczinski F, Hallak JE, Zuardi AW, Crippa JA, Quevedo J (2011). "Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala". Acta Neuropsychiatrica. 23 (5): 241–248. doi:10.1111/j.1601-5215.2011.00579.x. PMID 25379896.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Crippa, J.A., Derenusson, G.N., Ferrari, T.B., Wichert-Ana, L., Duran, F.L.S., Martin-Santos, R., Simoes, M.V., Bhattacharyya, S., Fusar-Poli, P., Atakan, Z., Filho, A.S. (9 September 2010). "Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report". Journal of Psychopharmacology. 25 (1): 121–130. doi:10.1177/0269881110379283. PMID 20829306. S2CID 23662893.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leweke, F.M., Piomelli, D., Pahlisch, F., Muhl, D., Gerth, C.W., Hoyer, C., Klosterkotter, J., Hellmich, M., Koethe, D. (20 March 2012). "Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia". Translational Psychiatry. 2 (3): e94. doi:10.1038/tp.2012.15. PMC 3316151. PMID 22832859.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Consroe, P., Laguna, J., Allender, J., Snider, S., Stern, L., Sandyk, R., Kennedy, K., Schram, K. (November 1991). "Controlled clinical trial of cannabidiol in Huntington's disease". Pharmacology Biochemistry and Behavior. 40 (3): 701–708. doi:10.1016/0091-3057(91)90386-G. PMID 1839644. S2CID 42926914.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Prud’homme, Mélissa; Cata, Romulus; Jutras-Aswad, Didier (2015). "Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence". Substance Abuse: Research and Treatment. 9.
- ^ Romney, Lee (13 September 2012). "On the frontier of medical pot to treat boy's epilepsy". Los Angeles Times.
- ^ Volkow, Nora D. (24 June 2015). "Cannabidiol: Barriers to Research and Potential Medical Benefits". U.S. Department of Health and Human Services. Retrieved 16 March 2016.
- ^ a b c Lee, Martin A. (18 February 2015). "CBD Misconceptions". www.projectcbd.org. Retrieved 15 March 2016.
- ^ Good, Alastair (26 October 2010). "Growing marijuana that won't get you high". The Daily Telegraph. London.
- ^ Fournier, G.; Beherec, O.; Bertucelli, S. (2003). "Intérêt du rapport Δ-9-THC / CBD dans le contrôle des cultures de chanvre industriel". Annales de Toxicologie Analytique. 15 (4): 250–259. doi:10.1051/ata/2003003.
- ^ a b Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, Gang DR, Weiblen GD, Dixon RA (2009). "Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany. 60 (13): 3715–3726. doi:10.1093/jxb/erp210. PMC 2736886. PMID 19581347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (August 2007). "Cannabidiol--recent advances". Chemistry & Biodiversity (Review). 4 (8): 1678–92. doi:10.1002/cbdv.200790147. PMID 17712814. S2CID 3689072.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pertwee RG (2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin". British Journal of Pharmacology. 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC 2219532. PMID 17828291.
- ^ Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, Egawa T, Kitamura Y, Uchida N, Nishimura R, Egashira N, Iwasaki K, Fujiwara M (2008). "Cannabidiol potentiates pharmacological effects of Δ9-tetrahydrocannabinol via CB1 receptor-dependent mechanism". Brain Research. 1188: 157–164. doi:10.1016/j.brainres.2007.09.090. PMID 18021759. S2CID 25082053.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alchimia Blog, Cannabinoids and their medicinal properties
- ^ Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007). "The orphan receptor GPR55 is a novel cannabinoid receptor". British Journal of Pharmacology. 152 (7): 1092–101. doi:10.1038/sj.bjp.0707460. PMC 2095107. PMID 17876302.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Russo EB, Burnett A, Hall B, Parker KK (August 2005). "Agonistic properties of cannabidiol at 5-HT1a receptors". Neurochemical Research. 30 (8): 1037–43. doi:10.1007/s11064-005-6978-1. PMID 16258853. S2CID 207222631.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SR (January 2010). "Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors". British Journal of Pharmacology. 159 (1): 122–8. doi:10.1111/j.1476-5381.2009.00521.x. PMC 2823358. PMID 20002102.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS (January 2009). "5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats". British Journal of Pharmacology. 156 (1): 181–8. doi:10.1111/j.1476-5381.2008.00046.x. PMC 2697769. PMID 19133999.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Campos AC, Guimarães FS (August 2008). "Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats". Psychopharmacology. 199 (2): 223–30. doi:10.1007/s00213-008-1168-x. PMID 18446323. S2CID 22749049.
- ^ Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M (May 2005). "Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism". Stroke; A Journal of Cerebral Circulation. 36 (5): 1077–82. doi:10.1161/01.STR.0000163083.59201.34. PMID 15845890. S2CID 6806707.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, Fujioka M, Abe K, Hasebe N, Egashira N, Iwasaki K, Fujiwara M (March 2007). "Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance". Neuropharmacology. 52 (4): 1079–87. doi:10.1016/j.neuropharm.2006.11.005. PMID 17320118. S2CID 23816947.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E (2006). "Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 372 (5): 354–361. doi:10.1007/s00210-006-0033-x. PMID 16489449. S2CID 4877869.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bisogno, T., Hanuš, L., DePetrocellis, L., Tchilibon, S., Ponde, D.E., Brandi, I., Moriello, A.S., Davis, J.B., Mechoulam, R., Marzo1, V.D. (October 2001). "Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide". British Journal of Pharmacology. 134 (4): 845–852. doi:10.1038/sj.bjp.0704327. PMC 1573017. PMID 1573017.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (August 1995). "Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain". Drug Metabolism and Disposition. 23 (8): 825–831. PMID 7493549.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (November 2011). "Cannabidiol potentiates Δ⁹-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats". Psychopharmacology. 218 (2): 443–457. doi:10.1007/s00213-011-2342-0. PMID 21667074. S2CID 6240926.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hunt CA, Jones RT, Herning RI, Bachman J (June 1981). "Evidence that Cannabidiol Does Not Significantly Alter the Pharmacokinetics of Tetrahydrocannabinol in Man". Journal of Pharmacokinetics and Biopharmaceutics. 9 (3): 245–260. doi:10.1007/BF01059266. PMID 6270295. S2CID 23737809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ United States Adopted Names Council: Statement on a nonproprietary name
- ^ "Fact Sheet — Sativex". Health Canada. Retrieved 16 May 2013.
- ^ GWPharma- Welcome
- ^ "Georgia doctors encouraged in study of medical marijuana". Retrieved 2015-10-08.
- ^ Jones PG, Falvello L, Kennard O, Sheldrick GM Mechoulam R (1977). "Cannabidiol". Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials. 33 (10): 3211–3214. doi:10.1107/S0567740877010577.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mechoulam R, Ben-Zvi Z, Gaoni Y (1968). "Hashish—XIII On the nature of the beam test". Tetrahedron. 24 (16): 5615–5624. doi:10.1016/0040-4020(68)88159-1. PMID 5732891.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gaoni Y, Mechoulam R (1966). "Hashish—VII The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- ^ Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A (1967). "Synthese und Chiralität des (−)-Cannabidiols". Helvetica Chimica Acta. 50 (2): 719–723. doi:10.1002/hlca.19670500235. PMID 5587099.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gaoni Y, Mechoulam R (1985). "Boron trifluoride etherate on alumuna — a modified Lewis acid reagent. An improved synthesis of cannabidiol". Tetrahedron Letters. 26 (8): 1083–1086. doi:10.1016/S0040-4039(00)98518-6.
- ^ Kobayashi Y, Takeuchi A, Wang YG (2006). "Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate". Organic Letters. 8 (13): 2699–2702. doi:10.1021/ol060692h. PMID 16774235.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Taura, F., Sirikantaramas, S., Shoyama, Y., Yoshikai, K., Shoyama, Y., Morimoto, S. (2007). "Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa". FEBS Letters. 581 (16): 2929–2934. doi:10.1016/j.febslet.2007.05.043. PMID 17544411. S2CID 20253070.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ https://www.legislation.gov.au/Details/F2016L00174
- ^ Controlled Drugs and Substances Act – Schedule II
- ^ "Cannabis-Derived Dravet Syndrome Drug Gets US Orphan Drug Approval". Nov 18, 2013. Retrieved 21 July 2015.
- ^ Szaflarski, Jerzy P.; Bebin, E. Martina (August 2014). "Cannabis, cannabidiol, and epilepsy — From receptors to clinical response". Epilepsy & Behavior. 41: 277–282. doi:10.1016/j.yebeh.2014.08.135. PMID 25282526. S2CID 20064410.
- ^ "An Overview of "Carly's Law": Alabama's CBD-extract only medical marijuana law". mpp.org. 2014. Retrieved 15 March 2016.
- ^ https://www.medicines.org.uk/emc/medicine/23262
- ^ "CosIng - Cosmetics - Health and Consumers - European Commission". ec.europa.eu. Retrieved 2016-04-11.
External links
[edit]- Project CBD Nonprofit educational service dedicated to promoting and publicizing research into the medical utility of cannabidiol.
 | This is a user sandbox of SettleGod. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
