User:Hana.m.elarabi/sandbox
 | This is a user sandbox of Hana.m.elarabi. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
 | This is a user sandbox of Hana.m.elarabi. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Janis Louie
[edit]Janis Louie (born 3 November 1971 in San Fransisco, California, U.S.A.)[1] is a Chemistry professor and Henry Eyring Fellow at The University of Utah. Louie contributes to the chemistry world with her research in inorganic, organic, and polymer chemistry.[2]
Schooling
[edit]Louie received her B.S. from University of California, Los Angeles in 1993.2 She then moved on to get her Ph.D. from Yale University for work under Professor John Hartwig in 1998.[3] In the years of 1998-2001 Louie was an NIH Postdoctoral Fellow at the California Institute of Technology.[2]
Life outside of chemistry
[edit]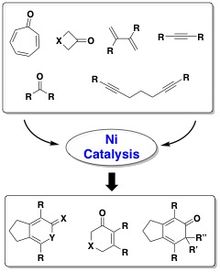
Besides being a chemistry professor and researcher at The University of Utah, Louie is married to her husband Chris and is a mother of triplets (one boy and two girls). While studying at UCLA during her undergraduate years, she was a cheerleader and slowly got herself into weightlifting. She continued weightlifting throughout graduate school and later career. In graduate school she discovered fitness competitions and while being an assistant professor at the University of Utah, competed in her first bodybuilding competition. Louie went on to compete in three bodybuilding competitions before competing in her first fitness competition, the TriFitness Challenge.[1]
Throughout her lifting career Louie has enjoyed many of the usual compound lifts such as bench, squat, and deadlift. But enjoys the squat, more specifically the pistol squat, and the pull up because so many muscles are being worked at the same time.[1]
Louie’s Research
[edit]Accessibility to structurally diverse organic compounds such as carbocycles and heterocycles are of great importance to the pharmaceutical and agrochemical industries.[4] However the synthesis of these types of compounds require harsh conditions such as high temperatures and pressures.[5] Dr. Louie’s research aims to mediate these transitions via metal catalyzed reactions, mainly involving nickel catalysis.[2] Dr. Louie and her team focuses on nickel-based systems not only because it is much less expensive than the more widely used palladium and platinum, but also because it offers a wider range of chemical activity,[4] such as nickel, being a more electropositive transition metal which allows it to undergo oxidative addition readily; oxidize Nickel and lessens the electron density around the atom itself.6 This allows for the cross-coupling of electrophiles to occur which is pivotal in the formation of carbocycles and heterocycles.[6] Dr. Loui and her colleagues further enhance the catalytic ability by combining nickel with N-heterocyclic carbene (NHC) ligands.[6] The NHC ligands are largely sterically hindered and electron donating, which allows improved and less harsh reactions conditions by expanding the chemical scope of the substrate.[6] (Taking all these chemical factors into account, the Ni/NHC catalyst can effectively couple diynes and nitriles to create pyridines using a hetero-oxidative coupling mechanism. Also, this nickel catalysis method (Figure 1) offers a wider range of substrates for which nickel can perform cyclo-additions on, substrates such as; vinylcyclopropanes, aldehydes, ketones, tropones, 3-azetidinones, and 3-oxetanones).[6]
Louie is also involved in the study and research of diversifying organometallic catalysts. Usually, an organometallic catalyst is only used for one specific type of reaction, but Louie has been

working on the development of these organometallic catalysts as to make them able to catalyze more than one specific type of reaction.[7] This may be done by using the same catalyst, or by using one catalyst that can be slightly altered into making a catalyst that is useful for another type of reaction.[8]
Louie also experimented with the production of 2-pyrones. She would use carbon dioxide mixed with different diynes to prepare different 2-pyrones.[9] The simple reaction for the formation of 2-pyrones is shown in Figure [null 2] .
Louie’s nickel imidazolyidene complexes help progress the cyclization of terminal and internal functional group like aryl and alkyl isocyanates in a rather mild manner as opposed to synthetic synthesis.[10]
● N.D. Staudaher, J. Lovelace, M. P. Johnson, J. Louie “Preparation of Aryl Alkyl Ketenes” Org. Synth. 2016.
● A. Thakur, J. Evangelista, J. Louie “An in situ Ni-Catalyzed Approach to Substituted Piperidones” J. Org. Chem. 2015, 80, 9951-9958.
● A. Thakur, J. Louie “Advances in Nickel-Catalyzed Cycloaddition Reactions To Construct Carbocycles and Heterocycles” Acc. Chem. Res. 2015, 48, 2534-2365; Invited Contribution to a Special Issue (Earth Abundant Metals in Homogeneous Catalysis).
● Y. Zhong, N. A. Spahn, R. M. Stolley, M. H. Nguyen, J. Louie “3,5-Disubstituted-2-Aminopyridines via Ni-catalyzed Cycloaddition of Terminal Alkynes and Cyanamides” Synlett 2015, 26, 307-312; Invited Contribution to a Special Issue (Cluster Report on Catalysis with Sustainable Metals).
● P. Kumar, A. Thakur, X. Hong, K. N. Houk, J. Louie “[Ni(NHC)]-catalyzed Cycloaddition of Diynes and Tropone: Apparent Enone Cycloaddition Involving an 8p Insertion” J. Am. Chem. Soc. 2014, 136, 17844-17851.
● N. D. Staudaher, R. M. Stolley, J. Louie “Synthesis, Mechanism of Formation, and Catalytic Activity of Xantphos Nickel p-Complexes” Chem. Commun. 2014, 50, 15577-15580.
● A. Thakur, M. E. Facer, J. Louie “Nickel Catalyzed Cycloaddition of 1,3-Dienes with 3-Azetidinones and 3-Oxetanes” Angew. Chem. Int. Ed. 2013, 52, 12161-12165. PMID 24573793; PMC 4113093.
● R. M. Stolley, H. A. Duong, J. Louie “Mechanistic Evaluation of the Ni(IPr)2-Catalyzed Cycloaddition of Alkynes and Nitriles to Afford Pyridines: Evidence for the Formation of a Key h1-Ni(IPr)2(RCN) Intermediate” Organometallics 2013, 32, 4952-4960. PMID 25214702; PMC 4159214.
● T. K. Lane, M. H. Nguyen, N. Spahn, J. Louie “The Iron-Catalysed Construction of 2-Aminopyrimidines from Alkynenitriles and Cyanamides” Chem. Commun. 2013, 49, 7735-7737. PMID 23877441; PMC 4144345.
● R. M. Stolley, H. A. Duong, D. R. Thomas, J. Louie “The Discovery of [Ni(IPr)RCN]2 Species and their Role as Cycloaddition Catalyts for the Formation of Pyridines” J. Am. Chem. Soc. 2012, 134, 15154-15162. PMID 22917161; PMC 3480329.
● T. K. Lane, B. R. D’Souza, J. Louie “2-Aminopyridines from Iron-Catalyzed Cycloaddition of Diynes and Cyanamides” J. Org. Chem. 2012, 77, 7555-7563. PMID 22845666; PMC 3480319.
● P. Kumar, K. Zhang, J. Louie “An Expeditious Route to Eight-Membered Heterocycles by Nickel-Catalyzed Cycloaddition: Low-Temperature Csp2-Csp3 Bond Cleavage” Angew. Chem. Int. Ed. 2012, 51, 8602-8606. PMID 22806996; PMC 3557805.
● P. Kumar, J. Louie “A Single Step Approach to Highly Substituted Piperidines Via Ni-Catalyzed β-Carbon Elimination” Org. Lett. 2012, 14, 2026-2029; Synfacts Highlight 2012, 8(7), 0715;
● Synfacts Highlight 2012, 8(9), 0949. PMID 22468962; PMC 4138124.
● R. M. Stolley, W. X. Guo, J. Louie “Palladium-Catalyzed Cross-Coupling of Cyanamides” Org. Lett. 2012, 14, 322-325; C & E News December 19, 2011. PMID 22142553; PMC 4113087.
● T. N. Tekavec, J. Louie “Nickel-Catalyzed Cycloadditions of Unsaturated Hydrocarbons, Aldehydes, and Ketones” J. Org. Chem. 2008, 73, 2641-2648. PMID 18318544; PMC 4144363.
● Henry Erying Assistant Professorship 2004
● NSF Faculty Early CAREER Development Award, 2004–2009
● ACS/Dreyfus PROGRESS “Rising Stars” Lectureship, 2005–2006
● Camille and Henry Dreyfus Teacher-Scholar Award, 2005–2010
● Alfred P. Sloan Award 2006
● CS Arthur C. Cope Scholar Award 2007
● Fellow of the American Association for the Advancement of Science, 2012
● Kavli Foundation Fellow, 2012
● Sigma Chi Beta Epsilon Chapter Teacher Appreciation Award, 2015
References
[edit]- ^ a b c Personal communication
- ^ a b c d e The University of Utah. "Janis Louie." Department of Chemistry - The University of Utah. The University of Utah, 12 July 2016. Web. 09 May 2017. [1]
- ^ Louie, Janis, Michael S. Driver, Blake C. Hamann, and John F. Hartwig. "Palladium-Catalyzed Amination of Aryl Triflates and Importance of Triflate Addition Rate." The Journal of Organic Chemistry 62.5 (1997): 1268-273. Web.[2]
- ^ a b Gurjar, Mukund K., Somu V. Ravindranadh, Kuppusamy Sankar, Sukhen Karmakar, Joseph Cherian, and Mukund S. Chorghade. "Synthesis of Spirocycles via Ring Closing Metathesis of Heterocycles Carrying Gem-Diallyl Substituents Obtained via Ring Opening of (Halomethyl)cyclopropanes with Allyltributyltin." ChemInform 34.34 (2003): n. pag. Web.[3]
- ^ Louie, Janis, and Robert H. Grubbs. "Metathesis of Electron-Rich Olefins:Â Structure and Reactivity of Electron-Rich Carbene Complexes." Organometallics 21.11 (2002): 2153-164. Web.[4]
- ^ a b c d Thakur, Ashish, and Janis Louie. "Advances in Nickel-Catalyzed Cycloaddition Reactions To Construct Carbocycles and Heterocycles." Accounts of Chemical Research 48.8 (2015): 2354-365. Web.[5]
- ^ Louie, Janis, Christopher Bielawski, and Robert Grubbs. "Tandem Catalysis: The Sequential Mediation of Olefin Metathesis, Hydrogenation, and Hydrogen Transfer with Single-Component Ru Complexes." Journal of the American Chemical Society 123.45 (2001): 11312-1313. ACS Publications. ACS Publications. Web. 13 May 2017.[6]
- ^ Louie, Janis, John E. Gibby, Marc V. Farnworth, and Thomas N. Tekavec. "Efficient Nickel-Catalyzed [2 2 2] Cycloaddition of CO2and Diynes." Journal of the American Chemical Society 124.51 (2002): 15188-5189. Web.[7]
- ^ Duong, Hung A., Michael J. Cross, and Janis Louie. "Nickel-Catalyzed Cycloaddition of Alkynes and Isocyanates." Journal of the American Chemical Society 126.37 (2004): 11438-1439. Web.[8]
- ^ Zhang, Kainan, Martin Conda-Sheridan, Shayna R. Cooke, and Janis Louie. "N-Heterocyclic Carbene Bound Nickel(I) Complexes and Their Roles in Catalysis." Organometallics 30.9 (2011): 2546-552. Web. [9]
