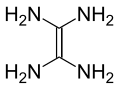
Tetraaminoethylene
Appearance
| |
| Names | |
|---|---|
| Systematic IUPAC name
Ethene-1,1,2,2-tetramine[1] | |
| Other names
Ethylenetetraamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H8N4 | |
| Molar mass | 88.114 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In organic chemistry, tetraaminoethylene is a hypothetical, organic compound with formula C2N4H8 or (H2N)2C=C(NH2)2. Like all polyamines that are geminal, this compound has never been synthesised and is believed to be extremely unstable.[2]
However, there are many stable compounds that can be viewed as derivatives of tetraaminoethylene, with various organic functional groups substituted for some or all hydrogen atoms. These compounds, which have the general formula (R2N)2C=C(NR2)2, are collectively called tetraaminoethylenes.
Tetraaminoethylenes are important in organic chemistry as dimers of diaminocarbenes, a type of stable carbene with the general formula (R2N)2C:.
Reactions
[edit]- Tetraaminoethylenes react with acids to give formamidinium salts.
- Tetraaminoethylenes react with oxygen to give urea derivatives (R2N)2C=O. A notorious example is the spontaneous reaction of Tetrakis(dimethylamino)ethylene ((H3C)2N)2C=C(N(CH3)2)2 in air with emission of a green-blue light, which was used by downed US Navy pilots to signal for help in World War II.
References
[edit]- ^ "CID 17899866 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Stephen A. Lawrence (2004), Amines: synthesis, properties and applications. Cambridge University Press. ISBN 0-521-78284-8, 371 pages.

