Tetrakis(dimethylamino)ethylene

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N1,N1,N′1,N′1,N2,N2,N′2,N′2-Octamethylethene-1,1,2,2-tetramine | |||
| Other names
Octamethyl-ethenetetramine
TDAE | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.012.398 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H24N4 | |||
| Molar mass | 200.330 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 0.861 g/cm3 (25 °C) | ||
| Melting point | −4 °C (25 °F; 269 K) | ||
| Boiling point | 59 °C (0.9 mm Hg) | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H226, H314 | |||
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P235, P405, P501 | |||
| Flash point | 53 °C (127 °F; 326 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tetrakis(dimethylamino)ethylene (TDAE) is an organic compound with the formula ((CH3)2N)2C=C(N(CH3)2)2, It is a colorless liquid. It is classified as an enamine. Primary and secondary enamines tend to isomerize, but tertiary enamines are kinetically stable.[1] One unusual feature of TDAE is that it is a tetra-enamine. The pi-donating tendency of the amine groups strongly enhances the basicity of the molecule, which does exhibit properties of a typical alkene.[2]
Reactions
[edit]TDAE reacts with oxygen in a chemiluminescent reaction to give tetramethylurea.[3][4] The initial intermediate is (2+2) adduct, a 1,2-dioxetane. This species decomposes to electronically excited tetramethylurea. This returns to the ground state is accompanied by emission of green light with a maximum at 515 nm.[5]

TDAE is an electron donor with E = 1.06 V vs Fc+/0.[6]
TDAE has been examined as a reductant in coupling reactions.[7]
As one of many of examples of its redox behavior forms a charge-transfer salt with buckminsterfullerene:[8]
- C2(N(CH3)2)4 + C60 → [C2(N(CH3)2)4+][C60−] Oxidation affords a dication.
Structure
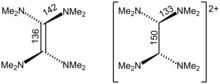
[edit]Crystallographic analysis show that TDAE is a highly distorted alkene, the dihedral angle for the two N2C termini is 28°. The C=C distance is alkene-like, 135 pm. The nearly isostructural tetraisopropylethylene also has a C=C distance of 135 pm, but its C6 core is planar. In contrast, [TDAE]2+ is an alkane with multi-C-N bonds.[9]
Synthesis
[edit]It is available by pyrolysis of tris(dimethylamino)methane by pyrolysis[10] or from chlorotrifluoroethene and dimethylamine.[11]
References
[edit]- ^ Spitz, Cédric; Terme, Thierry; Vanelle, Patrice (2023). "1,1,2,2-Tetrakis(dimethylamino)ethene". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X. hdl:10261/236866. ISBN 978-0-471-93623-7.
- ^ David M. Lemal (1968). "Tetraaminoethylenes". In Saul Patai (ed.). The Amino Group. Patai's Chemistry of Functional Groups. pp. 701–748. doi:10.1002/9780470771082. ISBN 9780470771082.
- ^ H.E. Winberg; J.R. Downing; D.D. Coffman (1965), "The Chemiluminescence of Tetrakis(dimethylamino)ethylene", J. Am. Chem. Soc., vol. 87, no. 9, pp. 2054–2055, doi:10.1021/ja01087a039
- ^ "Chemilumineszenz von TDAE" (in German). illumina-chemie.de. 2014-08-08. Retrieved 2016-08-22.
- ^ H.E. Winberg; J. R. Downing; D. D. Coffman (1965). "The Chemiluminescence of Tetrakis(dimethylamino)ethylene". J. Am. Chem. Soc. 87 (9): 2054–2055. doi:10.1021/ja01087a039.
- ^ Broggi, Julie; Terme, Thierry; Vanelle, Patrice (2014-01-07). "Organic Electron Donors as Powerful Single-Electron Reducing Agents in Organic Synthesis" (PDF). Angewandte Chemie International Edition. 53 (2): 384–413. doi:10.1002/anie.201209060. PMID 24273111.
- ^ Kuroboshi, Manabu; Waki, Yoko; Tanaka, Hideo (2003). "Palladium-Catalyzed Tetrakis(dimethylamino)ethylene-Promoted Reductive Coupling of Aryl Halides". The Journal of Organic Chemistry. 68 (10): 3938–3942. doi:10.1021/jo0207473. PMID 12737575.
- ^ Allemand PM, Khemani KC, Koch A, et al. (1991). "Organic Molecular Soft Ferromagnetism in a Fullerene". Science. 253 (5017): 301–302. Bibcode:1991Sci...253..301A. doi:10.1126/science.253.5017.301. PMID 17794696. S2CID 19561675.
- ^ a b Bock, Hans; Borrmann, Horst; Havlas, Zdenek; et al. (1991). "Tetrakis(dimethylamino)ethene: An Extremely Electron-Rich Molecule with Unusual Structure both in the Crystal and in the Gas Phase". Angewandte Chemie International Edition in English. 30 (12): 1678–1681. doi:10.1002/anie.199116781.
- ^ H. Weingarten; W.A. White (1966), "Synthesis of Tetrakis(dimethylamino)ethylene", J. Org. Chem., vol. 31, no. 10, pp. 3427–3428, doi:10.1021/jo01348a520
- ^ US 3293299, H. Boden, "Process for making tetrakis(dimethylamino)ethylene", published 1966-12-20, assigned to E.I. du Pont de Nemours and Co.



