Template:Infobox drug/testcases-warning
Appearance
| This is the template test cases page for the sandbox of Template:Infobox drug. to update the examples. If there are many examples of a complicated template, later ones may break due to limits in MediaWiki; see the HTML comment "NewPP limit report" in the rendered page. You can also use Special:ExpandTemplates to examine the results of template uses. You can test how this page looks in the different skins and parsers with these links: |
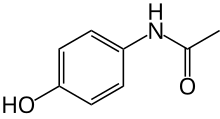
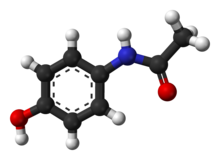
Paracetamol
[edit]| {{Infobox drug}} | {{Infobox drug/sandbox}} | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ^ International Drug Names
- ^ "Acetaminophen Use During Pregnancy". Drugs.com. 14 June 2019. Archived from the original on 9 March 2020. Retrieved 25 February 2020.
- ^ "Regulatory Decision Summary – Acetaminophen Injection". Health Canada. 23 October 2014. Archived from the original on 7 June 2022. Retrieved 7 June 2022.
- ^ Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine (2015). Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J (eds.). Acute Pain Management: Scientific Evidence (4th ed.). Melbourne: Australian and New Zealand College of Anaesthetists (ANZCA), Faculty of Pain Medicine (FPM). ISBN 978-0-9873236-7-5. Archived from the original (PDF) on 31 July 2019. Retrieved 28 October 2019.
- ^ a b c Forrest JA, Clements JA, Prescott LF (1982). "Clinical pharmacokinetics of paracetamol". Clin Pharmacokinet. 7 (2): 93–107. doi:10.2165/00003088-198207020-00001. PMID 7039926. S2CID 20946160.
- ^ "Codapane Forte Paracetamol and codeine phosphate product information" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 29 April 2013. Archived from the original on 6 February 2016. Retrieved 10 May 2014.
- ^ "Acetaminophen Pathway (therapeutic doses), Pharmacokinetics". Archived from the original on 4 March 2016. Retrieved 13 January 2016.
- ^ a b Pickering G, Macian N, Libert F, Cardot JM, Coissard S, Perovitch P, Maury M, Dubray C (September 2014). "Buccal acetaminophen provides fast analgesia: two randomized clinical trials in healthy volunteers". Drug Design, Development and Therapy. 8: 1621–1627. doi:10.2147/DDDT.S63476. PMC 4189711. PMID 25302017.
In postoperative conditions for acute pain of mild to moderate intensity, the quickest reported time to onset of analgesia with APAP is 8 minutes9 for the iv route and 37 minutes6 for the oral route.
- ^ Karthikeyan M, Glen RC, Bender A (2005). "General Melting Point Prediction Based on a Diverse Compound Data Set and Artificial Neural Networks". Journal of Chemical Information and Modeling. 45 (3): 581–590. doi:10.1021/ci0500132. PMID 15921448.
- ^ "melting point data for paracetamol". Lxsrv7.oru.edu. Archived from the original on 30 June 2012. Retrieved 19 March 2011.
- ^ a b c d e Granberg RA, Rasmuson AC (1999). "Solubility of paracetamol in pure solvents". Journal of Chemical & Engineering Data. 44 (6): 1391–95. doi:10.1021/je990124v.
Brincidofovir
[edit]| {{Infobox drug}} | {{Infobox drug/sandbox}} | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ^ "FDA approves drug to treat smallpox". U.S. Food and Drug Administration (FDA). 4 June 2021. Archived from the original on 8 June 2021. Retrieved 7 June 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Albendazole
[edit]| {{Infobox drug}} | {{Infobox drug/sandbox}} | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ^ Plumb DC (2011). "Albendazole". Plumb's Veterinary Drug Handbook (7th ed.). Stockholm, Wisconsin; Ames, Iowa: Wiley. pp. 19–21. ISBN 978-0-470-95964-0.
- ^ a b c d "Albenza, (albendazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on March 1, 2014. Retrieved February 25, 2014.