Wikipedia talk:WikiProject Pharmacology
| This is the talk page for discussing WikiProject Pharmacology and anything related to its purposes and tasks. |
|
| Archives: Index, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17Auto-archiving period: 3 months |
| This project page does not require a rating on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||
| ||||||||
| WikiProject Pharmacology was featured in a WikiProject Report in the Signpost on 17 January 2009. |
|
|
This page has archives. Sections older than 90 days may be automatically archived by Lowercase sigmabot III when more than 10 sections are present. |
Finding "undocumented" compounds
[edit]Hi, I'm not sure if this is the right place to ask this.
I've seen a lot of pharmacology navboxes (such as muscarinic receptor modulators, for an example), lots of them contain a lot of drugs that are not really known (a lot of them are redlinks), which is what I mean by "undocumented compounds"
I was just curious if anyone knew how to find names of these compounds (like specific keywords in a search engine)
Because so far I've been creating a lot of articles that were redlinks on the navboxes, but I'd love to add new compounds to said templates.
So if anyone knew how to find lists (or something similar) with names of investigational drugs (names like "ADX-71441") that would be really great Themonkey942 (talk) 22:29, 7 September 2024 (UTC)
Importance ratings need review
[edit]An editor first labeled Legality of cannabis as top-importance for this group, and after I removed the rating, has changed it to high-importance. Please decide for yourselves how you'd like to have it assessed.
I've seen a couple of editors "upgrade" their favorite subjects, perhaps in the mistaken belief that this will result in more editors working on the articles. I specifically suggest taking a look at what's in Category:Top-importance pharmacology articles and Category:High-importance pharmacology articles in particular, to see whether any spammers have been at work. WhatamIdoing (talk) 04:41, 26 September 2024 (UTC)
Dose and dosage
[edit]Is there a good, well-sourced page that describes the technical difference between dose and dosage? WhatamIdoing (talk) 17:52, 30 September 2024 (UTC)
- Apparently we did not have such an article, but I created dosage (pharmacology) which hopefully is a start. Boghog (talk) 18:58, 30 September 2024 (UTC)
- Thanks for creating that. WhatamIdoing (talk) 19:41, 30 September 2024 (UTC)
- Perhaps dosage (pharmacology) should be merged into Medical prescription. Thoughts? Boghog (talk) 19:48, 30 September 2024 (UTC)
- I'm inclined not to make a hasty decision (either way). Part of this is because I generally prefer merging, but other people tell me that a short, focused article is better for mobile users (which is most of our page views).
- I did some copyediting on Dose (biochemistry). WhatamIdoing (talk) 20:27, 30 September 2024 (UTC)
- In my opinion, a dosage and a prescription are two different (albeit related, of course) things. I'd be inclined not to merge, for that reason. --Tryptofish (talk) 20:44, 30 September 2024 (UTC)
- Perhaps dosage (pharmacology) should be merged into Medical prescription. Thoughts? Boghog (talk) 19:48, 30 September 2024 (UTC)
- Thanks for creating that. WhatamIdoing (talk) 19:41, 30 September 2024 (UTC)
- My view is that they are synonyms, and, with respect ot Boghog for work done, I don't think that we need that new page. Indeed, my reading of the key source in that new article is not consistent with the definition in the lede. Rowbotham et al (2019) actually say
we found dose and dosage to be used interchangeably. We recommend a distinction between these terms, with ‘dosage’ having the advantage of capturing change to amount ‘dispensed’ over time (in response to effects achieved). Dosage therefore acknowledges the inevitable dynamic and complexity of implementation
. So, the current state is that they are synonyms; their recommended use is a very subtle one that doesn't reflect the lede of the current article. My own UK-centric view is that dosage is an abomination that should be eliminated wherever it rears its ugly head, and that the term dose suffices for all civilized discussion. Given that bias, I suggest merging Dosage (pharmacology) to Dose (biochemistry), describing any differences in use on that page. Klbrain (talk) 21:56, 2 October 2024 (UTC)- Dorland's (2007) gives these definitions:
- dosage
- the determination and regulation of the size, frequency, and number of doses.
- dose
- quantity to be administered at one time, such as a specified amount of medication.
- The latter continues for three-quarters of a page to cover all the sub-definitions (e.g., total dose, daily dose, median effective dose...), but they don't use them as synonyms. That said, even though there is a verifiable technical distinction between the two, I think we could still merge the articles. It is not unusual for an article to have a note about the technical distinction between two words, especially when that distinction is more often ignored outside of technical documents. And to go even further than Klbrain, we might want to consider using the plain old English word amount when appropriate, for even greater clarity. WhatamIdoing (talk) 22:44, 2 October 2024 (UTC)
- See also Collins: dosage and Collins: dose. My understanding of the two terms matches what has been written in the Wikipedia articles. I suggest changing "given at one time" to "given at any one time" in case anyone thinks dose only refers to a one-off consumption. And that definition seems to match what Collins says is the British English use, as far as medicine is concerned. However, the US English use (citing Websters and their own definition in two sections) suggests some ambiguity with "the amount used in a dose" and "the amount of medicine to be given". At first this seems to indicate Americans are sometimes using "dosage" where they should say "dose" but then I considered what "amount" means, and it is cumulative.
- WAID mentions both "total dose" and "daily dose" which are different from an individual dose. A medicine of 100mg taken three times a day for two days has an individual dose of 100mg, daily dose of 300mg and total dose of 600mg. In each case the word dose is used, not dosage, though we might qualify use of the word that isn't referring to the individual dose if that isn't clear. Sometimes the word on its own can refer to the quantity consumed daily or in total. For example, if someone suffered ill effects from being on "too high a dose" of a medicine, this could be because they are taking it four times a day rather than two. If someone died from being given a "huge dose" of a medicine, this could be because the dose consumed over three days was ultimately fatal, whereas one individual dose might not. This all says to me that dose can be used to refer to an accumulation. The worse "dosage" couldn't be used for that at all.
- So perhaps in addition to "at any one time" (the individual dose) we could say "or in total over a period" (the daily dose or total dose). The American English usages in Collins would permit that definition. Have we got a source that say so more explicitly? -- Colin°Talk 08:00, 3 October 2024 (UTC)
- I see that in Dose (biochemistry)#Vaccines it says "Vaccinations are typically administered as liquids and dosed in milliliters" Clearly this isn't millilitres of vaccine. For example this Covid vaccine is a 0.3ml dose containing 30 micrograms of the vaccine. A liquid medicine such as Epilim liquid is 200mg/5ml. The dose in that case might refer to 400mg twice a day or 10ml twice a day or most precisely as 400mg as 10ml twice a day. One doesn't care about the size of a pill, though one might need to take two pills if the dose requires it.
- As the lead of of Dose (biochemistry) says, it can refer to the taking of some unit of medicine without anyone giving a size in mg or ml. For example this government report says "Up to 23 August 2022, 53 million people received a first dose of COVID-19 vaccine, 50 million received a second dose and 40 million received a third or booster dose."
- I wonder if the dose article could say more about how sometimes dose references the quantity of the containing liquid or pills rather than the amount of medicinal compound. "Take two paracetamol". "Draw up 0.3ml of covid vaccine from the vial". "Take 30ml of cough medicine". -- Colin°Talk 08:30, 3 October 2024 (UTC)
- I think that Dose (biochemistry), though not even close to our worst articles, is sufficiently weak that any editor who's looked in a dictionary/has a basic grasp of the concept could improve it through a quick copyedit. WhatamIdoing (talk) 19:54, 3 October 2024 (UTC)
- Dorland's (2007) gives these definitions:
- Look the drug up by full name in most wifi and ull find all that sort of info : 2600:1000:A121:5319:712C:16E4:EA57:57EE (talk) 22:27, 13 November 2024 (UTC)
Good article reassessment for Mephedrone
[edit]Mephedrone has been nominated for a good article reassessment. If you are interested in the discussion, please participate by adding your comments to the reassessment page. If concerns are not addressed during the review period, the good article status may be removed from the article. Z1720 (talk) 16:10, 12 October 2024 (UTC)
External links in list articles
[edit]I noticed the new list of investigational autism and pervasive developmental disorder drugs today, which is one of many articles submitted via AfC by the prolific IP editor 76.174.0.57. The list is clearly worthwhile but I do note that it has 89 external links in its main sections and only three actual references. This seems to go against the guidance at WP:ELLIST but is common in similar lists of interest to the Pharmacology Project. Some of the external links may be totally unnecessary, since there are valid wikilinks to existing articles where reference sources can be found. In other cases, it might be better to convert the EL to cites. Is this worth discussion and agreement on a consistent approach? Mike Turnbull (talk) 14:53, 28 October 2024 (UTC)
- Fine for the links to be turned into refs. It's mostly just that it's too overwhelming to do it myself. Especially considering that the lists require regular updating, which usually entails remaking them from scratch. Ideally turning the refs into links would be automated via a batch method somehow. But I'm not aware of a tool for that. (Perhaps User:Boghog might, since I see them templating a lot of bare URLs?) I will admit that it is also significantly easier to check the status of the drugs (e.g., many at once) when the cites are formatted as external links rather than as refs though. But if Wikipedia policy dictates that they should be formatted as refs then that's how they should be. – 76.174.0.57 (talk) 21:45, 28 October 2024 (UTC)
- I went ahead and converted the external links into citation tags. Although this might make them slightly harder to follow, templated citations could be useful when turning the red drug links into stubs. For anyone interested, pasting the following RegEx into the "search and replace" function of Wikipedia’s editing toolbar will convert the external links into refs containing bare URLs:
Search for: \[https(.*?)\]
Replace with: <ref>https\1</ref>
- In a second step, the WP:reFill (link) tool will convert the bare URLs into {{cite web}} templates. Boghog (talk) 06:51, 29 October 2024 (UTC)
- @Boghog Thanks for teaching me something new and useful! I'm going to apply that technique to the other lists I linked above. Two comments: 1) the search string needs to be
\[http(.*?)\]in older articles using http rather than https and 2) you had a typo in your replace string, which should be<ref>https$1</ref>Had me fooled for a moment! Mike Turnbull (talk) 13:24, 29 October 2024 (UTC)- @Mike Turnbul I forgot about non-secure URLs. Good catch. Alternatively, the search regex could be modified to
\[https?(.*?)\]. "s?" will match zero or one occurrence of "s", and hence will match both "http" and "https". The back reference designation for most regex parsers I am familiar with is "\1" which worked when I tested it. When I tried "$1", instead of inserting the captured URL, it inserted literally "$1". So I am a bit confused by your second statement. Boghog (talk) 13:59, 29 October 2024 (UTC)- @Boghog I've had little experience with regex searches but when I tried your original "\1" version this was the diff, which I reverted as it had added the "\" into the output. I then looked at the table in Regular_expression#Examples, where the second row on bracketed groups says that $1 can be used "later" to refer to the matched pattern. I then used the $ version successfully. My instinct is always to do the experiment and when something works I rarely worry about why it did! That entry in the table does say that some implementations use "\1". Mike Turnbull (talk) 14:41, 29 October 2024 (UTC)
- @Mike Turnbul I forgot about non-secure URLs. Good catch. Alternatively, the search regex could be modified to
- @Boghog Thanks for teaching me something new and useful! I'm going to apply that technique to the other lists I linked above. Two comments: 1) the search string needs to be
- Such a simple solution. Thanks Boghog and Mike Turnbull. Saved an otherwise huge amount of work. – 76.174.0.57 (talk) 21:08, 29 October 2024 (UTC)
Contra TAAR1 agonism as the mediator of amphetamine actions
[edit]It is widely stated or implied across the English Wikipedia that amphetamine and related monoamine releasing agents (MRAs) induce their effects by agonism of the trace amine-associated receptor 1 (TAAR1). This notion is generally presented as fact and is stated in many relevant Wikipedia articles (e.g., amphetamine, methamphetamine, dextroamphetamine, Adderall, lisdexamfetamine, MDMA, stimulant, trace amine, TAAR1, etc.). It is even presented in figures (e.g., Template:Amphetamine pharmacodynamics).
The promotion of this theory seems to have originated with User:Seppi333, who has done a large amount of seemingly excellent work on the amphetamine and related articles. User:Seppi333 cites studies published in the 2000s by Xie and Miller and colleagues who found that monoamine transporter (MAT) reversal and monoamine efflux induced by phenethylamine and amphetamines was dependent on TAAR1 activation in-vitro.[1][2][3][4] These findings would appear to substantiate the theory.
While I respect and appreciate User:Seppi333 and his hard work on the amphetamine-related content on Wikipedia, I have strong reservations about the TAAR1 activation theory and the widespread promotion of this theory on Wikipedia. The reasons for this are numerous, and include the following:
- TAAR1 is non-essential for amphetamine actions in vitro and in vivo: Amphetamines and other MRAs continue to induce monoamine efflux and other MRA-type actions like DAT internalization in vitro in human embryonic kidney 293 (HEK293) cells transfected with MATs.[5][6][7][8][9][10][11][12] This is notable as these non-neuronal cells do not express the TAAR1.[5] Likewise, amphetamines continue to increase monoamine release and produce stimulant-type behavioral effects in vivo in TAAR1 knockout mice (more on that below).[13][14][15]
- Many MRAs do not activate TAAR1 or do so very weakly: There are many MRAs that do not activate the human TAAR1 (EC50 > 30 μM). These include methcathinone, mephedrone, flephedrone, brephedrone, ephedrine, 4-methylamphetamine, PMA, 4-MTA, MDEA, MBDB, 5-APDB, 5-MAPDB, mCPP, TFMPP, and methylhexanamine (DMAA), among others.[16][5] Hence, TAAR1 agonism again appears to be non-essential for induction of monoamine release by MRAs. In addition, many amphetamines that do act as TAAR1 agonists, including amphetamine, methamphetamine, and MDMA, only activate the human TAAR1 at micromolar concentrations that are far higher than their nanomolar potencies for inducing monoamine release in vitro, and hence may not be pharmacologically relevant in humans.[16][17] Also, in contrast to many other MRAs acting as TAAR1 agonists, MDA and MDMA are notable in actually being weak partial agonists of the human TAAR1 (Emax ≈ 11–26%). As such, they seem more likely to be antagonist-like than agonist-like at the TAAR1.[16][18]
- TAAR1 signaling is antidopaminergic and anti-reinforcing: TAAR1 activation robustly inhibits the striatal dopaminergic and rewarding effects of amphetamines and of other reinforcing drugs like cocaine, opioids, and alcohol.[3][19][20][21][22][23][24] This has been shown with TAAR1 agonists[25][26] and with TAAR1 overexpression.[27] Moreover, TAAR1 knockout mice are supersensitive to the monoamine release and behavioral effects of stimulants and MDMA.[13][15][14][28] Accordingly, TAAR1 agonists like ulotaront do not have amphetamine-like effects in animals and show no misuse potential.[29] In addition, TAAR1 agonism produces robust aversive effects in animals.[30][31] Relatedly, it has been proposed that TAAR1 agonism by amphetamines serves to auto-inhibit and constrain their effects.[14] It has also been suggested by some researchers that lack of TAAR1 agonism with cathinones like mephedrone may enhance their reinforcing effects relative to amphetamines.[32] Due to their antidopaminergic and hence antipsychotic-like effects, TAAR1 agonists like ulotaront are being studied for treatment of schizophrenia and have reached late-stage clinical trials for this indication.[33][34] Similarly, TAAR1 agonists are being investigated for potential antagonistic therapy of psychostimulant addiction.[35][20][24]
- Experts on MRAs do not implicate TAAR1 signaling: Major literature reviews by top experts on MRAs, such as Richard B. Rothman and David J. Heal, state that the mechanism by which amphetamines induce monoamine release is unknown or poorly understood, and do not implicate TAAR1 activation.[36][37][38][39] Rothman and his group are notable in being prominent NIDA researchers and in having developed the major in-vitro assay that is used to evaluate the releasing actions of MRAs.[40] In my experience, it has been hard to find secondary source literature in general that presents TAAR1 activation as mediating amphetamine or MRA actions. It would seem to be an obscure idea in the literature.
- An expert critiques Wikipedia's TAAR1 MRA theory: Matthew Baggott, a major MDMA researcher, founder of Tactogen (a company developing novel MDMA-like MRAs as medicines), and collaborator with the Rothman/NIDA group, has criticized and discounted Wikipedia's TAAR1 theory of MRA action on social media.[41][18][42][17][43] He has provided various arguments against it in his posts, including several of the above points. The following quote gives an idea: "My take is that TAAR1 agonism is misunderstood, especially on Wikipedia, and it decreases the effects of stimulants. TAAR1 knockout mice have increased effects of stimulants (eg here) while mice that overexpress TAAR1 have less sensitivity (eg here). A TAAR1 agonist is potentially a novel way to decrease dopamine activity."[41]
I should note that TAAR1 agonism paired with concomitant uptake by MATs (i.e., MAT substrate activity) is a key part of the TAAR1 MRA theory and might help to explain differences between non-MRA TAAR1 agonists and amphetamine-type MRAs acting as TAAR1 agonists.[4] (The TAAR1 is an intracellular receptor, and uptake by MATs would in theory result in more potent activation of TAAR1 in the subset of neurons that co-express TAAR1 and MATs.) However, this still does not seem to account for many of the other findings that contradict the theory described above.
I have had reservations and suspicions about the TAAR1 and MRAs theory here on Wikipedia for years. However, I'm not an expert in this subject area and I thought I might be missing something. As a result, I opted not to raise my concerns. In any case, coming across Matthew Baggott's critiques of the theory on social media finally changed my mind and motivated me to act.
User:Seppi333 and any others, if there is something I'm missing, I'm open to being enlightened. But, considering all of the above, I don't think I'm wrong here. It appears to me that TAAR1 agonism may modulate the effects of MRAs, and theoretically might play some role in monoamine release induction in the case of some MRAs (per those Xie/Miller et al. in-vitro studies). However, it seems that the available research is overall strongly in conflict with the idea that TAAR1 activation is responsible or essential for monoamine release induced by amphetamine or MRAs generally.
Based on the above, I propose that a major clean up of the relevant content on Wikipedia may be in order.
Also pinging User:Professional Crastination, since they have done some editing on the relevant pages as well and may have input.
Thank you. – AlyInWikiWonderland (talk, contribs) 10:45, 13 December 2024 (UTC)
- Wikipedia doesn’t suggest TAAR1 is a mediator of the mode of action of MRAs, but hey if you want to dispute the fact that it’s not central to amphetamine’s pharmacology, I’ll make you and “Matthew Baggott” look like retards. Never call me out like this again. Seppi333 (Insert 2¢) 22:02, 13 December 2024 (UTC)
- Reminder: WP:CIV and WP:NPA. I didn't mean to infringe on your ego. This is about the content and its factual accuracy. And about whether said content reflects scientific consensus. I care about those things. And to me, something seems very off in this area. Please try to see it from others' perspectives.
- You state this:
Wikipedia doesn’t suggest TAAR1 is a mediator of the mode of action of MRAs, [...]
But the amphetamine, dextroamphetamine, methamphetamine, and many other relevant articles appear to implicate TAAR1 in mediating amphetamine-/MRA-induced monoamine reuptake inhibition and release (MAT internalization, reverse transport, and/or efflux). Many of these articles seem to present TAAR1 agonism as the foremost and key action of amphetamines, even before their well-accepted status as MRAs. Per everything I wrote above, there appear to be many strong points that go against TAAR1 mediation of amphetamine and MRA actions. Moreover, TAAR1 mediating the monoamine release induced by amphetamine and other MRAs does not appear to be widely accepted or scientific consensus, yet Wikipedia is currently presenting it like it is. As indicated by social media, many laypersons have the impression that TAAR1 mediates the MRA actions of amphetamines, and that appears to be because of Wikipedia. As far as I'm aware, you are responsible for most of the relevant Wikipedia content in this area. Something seems very wrong here to me, and apparently to others like Baggott (an expert on MRAs). The burden is on you to substantiate and explain this.
- You state this:
- I asked you about this topic years ago in private and IIRC you linked me to one of the Xie and Miller papers. While those studies do provide basic in-vitro mechanistic substantiation, those studies, in light of all of the contradicting findings, haven't been satisfactory for me on their own and I've continued to feel conflicted and confused. If I'm somehow mistaken about things, please explain them to me. As I mentioned, I would be happy to be enlightened. But it is very difficult for me to see how all of the counterpoints can be reconciled with what's currently presented on Wikipedia. Thus my raising of these concerns publicly and interest in refactoring the relevant content.
- Lastly, I'm not specifically contesting that TAAR1 agonism may be involved in and modulatory of amphetamine effects. I'm challenging the notion that TAAR1 agonism is key for amphetamine-/MRA-induced monoamine release (as well as MRA-type reuptake inhibition). And, by extension, for their consequent effects like psychostimulant or entactogen effects. (However, considering the micromolar EC50 values of amphetamine and other related MRAs for hTAAR1[16] and Baggott's comments in this area,[17][42][41] I do have some suspicions about whether TAAR1 agonism is indeed a significant action of amphetamine in humans at all as well. Is there any in-vivo primate or human evidence that demonstrates definitive involvement of TAAR1 agonism in amphetamine effects? Because if not, then I would relegate that idea to the status of an open question rather than proven fact also.) – AlyInWikiWonderland (talk, contribs) 04:45, 14 December 2024 (UTC)
- Sigh. If you actually read the articles, you'd realize its the drug's action at TAAR1 and VMAT2 TOGETHER that mediate its mechanism of action as an MRA. It is NOT TAAR1 alone, which is basically what every paper i've cited which mentions amphetamine and also states that TAAR1 agonists are anti-addictive says.
- I don't have any burden of proof to worry about. Nice attempt, though. Why? Amphetamine is an FA and if you try making bullshit changes like what you're implying, you'll be reverted by other people who are actually active on WP. I seriously think you're just trolling me, but if you're actually genuinely concerned, you need to read the papers a lot more closely. Seppi333 (Insert 2¢) 07:57, 14 December 2024 (UTC)
- For what it's worth, I apologize for coming off so angrily. Bad day. Edit: Sorry for being an asshole. I'll follow up more tomorrow. It seems like you are being genuine. Seppi333 (Insert 2¢) 08:18, 14 December 2024 (UTC)
- Alright. So per your account, it's TAAR1 and VMAT2 together (in addition to plasmalemmal MAT uptake/substrate activity) that allow for mediation of MRA actions. Aside from further adding to why MRAs acting as TAAR1 agonists and non-MRA TAAR1 agonists are different however, I don't see how this changes things re: the other points I raised (but what User:Professional Crastination said partly does; more on that below). Moreover, several notable MRAs, including phenmetrazine, phentermine, and benzylpiperazine, are inactive at VMAT2 per the Rothman group.[36][44] Others like mephedrone also show only weak VMAT2 activity.[45] Phenmetrazine is notably a robust NDRA with similar effects to amphetamine and methamphetamine. So VMAT2 activity would appear to be non-essential for marked monoamine release with MRAs. To me, this would seem to add another point against the theory. How would you explain that?
- I am indeed being genuine. Another reminder: WP:AGF. In any case, thank you for dialing things down. – AlyInWikiWonderland (talk, contribs) 00:27, 16 December 2024 (UTC)
- There are so many other possible mechanisms you are discounting simply to "disprove" how amphetamine works. Maybe they strongly upregulate DA synthesis prior to vesicular uptake. Perhaps they inhibit DA breakdown within the cytosol of DA neurons. Have all those and other theories been tested? Again, the only thing I really know is how AMPHETAMINE works, not MRAs in general. And there are so many possible mechanisms of action that would explain an increased cytosolic load of dopamine besides VMAT2 inhibition. Seppi333 (Insert 2¢) 10:28, 16 December 2024 (UTC)
- I am pointing out inconsistencies in your personal theory of how amphetamine works. Your suggestions for how to resolve this particular inconsistency involve additional assumptions and mechanisms for numerous MRAs. The weak VMAT2 activity of mephedrone notably also appears to extend to other cathinones, such as methcathinone and methylone.[46][47] A more parsimonious account is that VMAT2 inhibition/reversal and increased cytosol monoamine levels are not actually required for efficacious monoamine release with MRAs generally. Indeed, amphetamine can still release dopamine without VMAT2.[48] In fact, it appears that VMAT2 involvement in the actions of amphetamines is actually controversial and uncertain.[48][49][46]
- Interestingly, there is also a whole alternative model of amphetamine action involving promotion of exocytotic dopamine release and enhancement of phasic dopamine signaling based on in-vivo animal findings.[50][48][51] Per these researchers, this might actually be how amphetamine works at "low" doses (like those used therapeutically), and augmentation of DAT efflux and tonic dopaminergic signaling may be more relevant to high misused and/or toxic doses.[50][48][51] (The quite comprehensive Reith & Gnegy (2020) review notably covers this theory, yet, again, does not even mention your TAAR1 agonism theory.[48])
- If VMAT2 activity is also non-essential for induction of monoamine release and robust associated effects, then I feel that that seriously further calls into question your model involving TAAR1 agonism. I hope the preceding also further emphasizes how much uncertainty and disagreement there remains in the scientific community in general about how amphetamine and other MRAs act. I think you are being overconfident in your particular view and aren't giving other views proper consideration (both personally and in terms of Wikipedia content). The truth is that we don't have full understanding nor scientific consensus on how amphetamines work and different theories and postulated mechanisms are still being evaluated.
- I'll respond to the other comments here later, have to go for now. – AlyInWikiWonderland (talk, contribs)
- Hey everyone. I was pinged here so I'm going to post my input. I'm not sure where exactly I should place my first reply as discussion is already under way, but I'll place it under @Seppi333's most recent reply because I think this will serve as an extension of it, if only because I believe he will benefit from some R&R in response to whatever happened off-wiki today and in the meantime it will give @AlyInWikiWonderland something to consider Re:
TAAR1 and amphetamine
. - Before I continue, I'd like to apologise in advance for the late reply. I was at The Killers last night and then went to the zoo with my partner today. Secondly, I'm also apologising in advance for any awkwardly worded sentences I may have written in this reply; I haven't had the privilege of the best quality sleep over the last few nights, and even though I take a medication that is sometimes used to treat excessive daytime sleepiness, I'm still very capable of making fatigue-related task errors that seem unfathomable in real time :P. Don't worry though, I love my ~8-9 hours of sleep a night as much as the next person.
- Lastly, I should also point out that much of my edits on the amphetamine and methamphetamine articles have been concentrated on the uses/medical section. Seppi wrote much, if not all of the pharmacology section, so I imagine they would be the best person to clarify any concerns you have about the coverage of TAAR1 by doing what they do best a la supplying a technically nuanced essay on the subject.
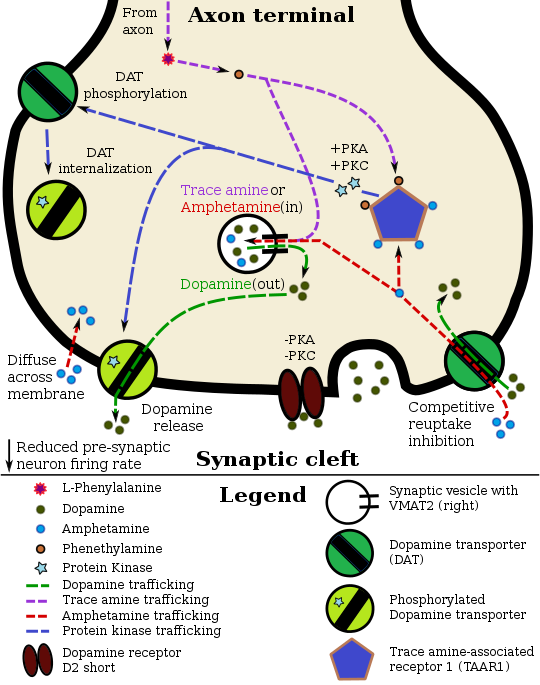
- The idea that TAAR1’s involvement is overstated in the coverage of pharmacology on the amphetamine wikipedia article isn't reflective of the available evidence. TAAR1 is unequivocally a major intracellular target of amphetamine, but it does not act in isolation. Amphetamine-induced monoamine efflux is mediated through multiple signalling cascades that regulate DAT function, including pathways that happen to be initiated by TAAR1 activation and protein kinase activity downstream, as well as of other possible unidentified targets in monoamine neurons (e.g., CAMKII).
- For context, TAAR1 activation triggers PKA- and PKC-dependent phosphorylation of DAT, which facilitates reverse transport/MA efflux and transporter internalisation respectively (NB: whether DAT is internalised or reversed is dependent on the residues phosphorylated). RhoA activation, which leads to ROCK-dependent internalisation of DAT and EAAT3, occurs downstream of TAAR1. This RhoA-driven process is transient because PKA inactivates RhoA after a short period, at which point PKA-mediated DAT internalisation dominates. Importantly, these cascades do not occur spontaneously. Amphetamine induces efflux through DAT via signalling cascades that involve kinase-dependent transporter phosphorylation. Amphetamine's entrance into the plasma membrane — whether by DAT uptake or passive diffusion — does not directly cause transporter phosphorylation. Rather, it signals through intracellular biomolecular targets, such as TAAR1 or others that are yet-to-be identified, to activate protein kinases that phosphorylate DAT via that signalling cascade. Amphetamine itself does not donate a phosphate group to the protein.
- Also, to expand on what Seppi stated Re:
" its the drug's action at TAAR1 and VMAT2 TOGETHER that mediate its mechanism of action as an MRA"
: cytosolic DA that's derived from amphetamine's action at VMAT2 significantly amplifies the magnitude of DA involved in DAT-mediated efflux, but without intracellular signalling through a kinase (e.g., TAAR1 --> PKC or unidentified target ----> CAMKII-alpha), there is no known mechanism that links VMAT2-driven DA release to DAT phosphorylation. If amphetamine had no effect on VMAT2, DAT would still be phosphorylated, and DA efflux would occur, but the magnitude would naturally be mardkedly reduced relative to the scenario where amphetamine interacts with VMAT2 and causes the collapse of the vesicular pH gradiant as a consequence. - Below is a table written put together by Seppi333 that encomapsses much of the research on amphetamine and its (known) kinase-mediated signalling cascades based on the avaliable evidence as of 2019. This table is supposed to support an updated model of the amphetamine pharmacodynamics table, but that's yet to come. As an aside, I was actually going to take a crack at updating the SVG for that template a few weeks ago, as my more notable edits in 2024 have been concerned with significantly expanding coverage of amphetamine and its transcluded articles so that they're up-to-date (e.g., adding content on the efficacy of its non-ADHD medical indications narcolepsy and binge eating disorder). However, in consistency with my relevant-as-ever username, I placed that at the back of my to-do pile if only because graphic design is not my passion.
- Hey everyone. I was pinged here so I'm going to post my input. I'm not sure where exactly I should place my first reply as discussion is already under way, but I'll place it under @Seppi333's most recent reply because I think this will serve as an extension of it, if only because I believe he will benefit from some R&R in response to whatever happened off-wiki today and in the meantime it will give @AlyInWikiWonderland something to consider Re:
Effects of amphetamine on membrane transport proteins in dopamine neurons Biological target
of amphetamineSecondary effector
protein kinasePhosphorylated
transporterEffect on transporter function Effect on neurotransmission Sources Unidentified CAMKIIα DAT Reverse transport of dopamine Dopamine efflux into synaptic cleft [52][53][54] TAAR1 ROCK† DAT Transporter internalization Dopamine reuptake inhibition [55][56][57] TAAR1 ROCK† EAAT3 Transporter internalization Glutamate reuptake inhibition [55][56][57] TAAR1 PKA DAT Transporter internalization Dopamine reuptake inhibition [58][59] TAAR1 PKC DAT Reverse transport of dopamine
Transporter internalizationDopamine efflux into synaptic cleft
Dopamine reuptake inhibition[53][58][59] †Note: ROCK-mediated transporter internalization is transient due to the inactivation of RhoA (which activates ROCK) by PKA. [60][56][57]
- Re:
TAAR1 knockout models
- The argument that TAAR1 KO models demonstrate TAAR1’s irrelevance ignores the fact that TAAR1 and D2 autoreceptors interact dynamically to regulate dopamine signalling. TAAR1-D2 heterodimerisation is eliminated in knockout models, and D2 receptor expression is markedly upregulated in the absence of TAAR1. These compensatory changes completely disrupt the normal dynamics of dopamine release and reuptake. As a result, TAAR1 KO experiments do not provide a controlled comparison of TAAR1 function. They do demonstrate how removing TAAR1 borks the balance of the release/reuptake system, though.
- With all that said, I can't think of anything else to add right now. I think I'm going to got take a nap and check back in on this either tomorrow or the day after. :P Professional Crastination (talk) 10:29, 14 December 2024 (UTC)
- agree with Seppi333(short but to the point)--Ozzie10aaaa (talk) 13:32, 14 December 2024 (UTC)
- Re:
- Thanks for the cordial and extensive reply User:Professional Crastination, I appreciate it. Your response helped clarify some important things for me about what you and User:Seppi333 are claiming. The main takeaway for me is that you two believe that amphetamine (and/or other MRAs) do not induce MAT monoamine efflux via TAAR1 alone but by multiple intracellular targets (e.g., TAAR1, unidentified target -> CAMKII, etc.). This is important since it would seem to explain, at least theoretically, (1) how amphetamine could continue to induce monoamine release (albeit seemingly less potently) without TAAR1 in vitro; (2) how amphetamine could still be active in TAAR1 KO mice in vivo (aside from additionally acting as a monoamine reuptake inhibitor); and (3) how other MRAs without hTAAR1 agonism could be active as MRAs in spite of lack of TAAR1 activation. This would seemingly obviate a few of my points. Would you and User:Seppi333 agree with that interpretation?
- I suppose then a critique I have is that this is not well-represented in the relevant content on Wikipedia in many places, like the dextroamphetamine, Adderall, lisdexamfetamine, and monoamine transporter pages (which make no mention of pathways other than TAAR1 such as CAMKII in their text, though CAMKII is mentioned in that transcluded figure). I feel like the relevant parts of these articles in general should start with something like "amphetamine appears to induce monoamine efflux via multiple known targets, including [...]". Right now, they start with TAAR1 agonism, and CAMKII is left almost as an afterthought. Relatedly, it's unclear to me why TAAR1 agonism should be highlighted as preferential, aside simply from its status of being of known identity. If many other MRAs are not hTAAR1 agonists yet are still very efficacious MRAs, if amphetamine is still such a robust releaser in TAAR1 knockout mice, and so on, then I feel like that all implies that the CAMKII pathway (and/or any other pathways) may be a lot more important and TAAR1 less so? Moreover, are there any other possible (known or unknown) pathways besides these two (for MAT monoamine efflux specifically)? As an example, I noticed that amphetamine and mephedrone have been implicated in mediating monoamine efflux through OCT3 (SLC22A3), and this seems to explain paradoxical synergy between MDPV and mephedrone.[32][61][62]
- Re:
TAAR1 is unequivocally a major intracellular target of amphetamine, [...]
. I understand that you and User:Seppi333 feel this way. And you might be correct. But this is an uncited assertion. What are the hard sources/evidence to show that this is true? I'm particularly preoccupied now with the micromolar EC50 values of amphetamine and methamphetamine at the hTAAR1, which are seemingly not compatible with TAAR1 agonism being a significant action of these agents at clinical doses in humans. Indeed, I've seen multiple papers imply that amphetamine and/or methamphetamine probably aren't significant TAAR1 agonists at therapeutic doses in humans but TAAR1 agonism might become significant in the context of methamphetamine addiction with extreme dose escalation and tolerance (resulting in far higher concentrations than usual that could potentially activate the TAAR1).[63][64] (It's notable that David K. Grandy is an author of these papers. He has also coauthored a relevant 2016 review with Miller.)[65] You can show that TAAR1 agonism produces monoamine release in vitro, but it's another thing to show that that's actually relevant in vivo.
- Re:
- Re: VMAT2, I mentioned above to User:Seppi333 that several notable MRAs are inactive at VMAT2 yet remain robust MRAs with effects similar to those of amphetamine and methamphetamine, for instance phenmetrazine. How do you explain this?
- Re: TAAR1 KO mice, yes, that does seem to make sense. And it makes more sense if amphetamine induces MAT monoamine release by multiple targets, not merely by TAAR1 agonism, because then it can still be active in KO mice. That said, an interesting study I came across has me questioning your explanation.[66] It found that TAAR1 KO amplified dopamine release and hyperlocomotion induced by MDMA (a TAAR1 agonist) relative to WT in vivo, as we see with (meth)amphetamine, whereas dopamine release induced by the closely related MRA para-chloroamphetamine (PCA) (not a TAAR1 agonist) was not different between TAAR1 KO and WT in vivo.[66] Moreover, addition of a TAAR1 agonist (o-PIT) to PCA blunted PCA-induced dopamine release both in vivo and in vitro in synaptosomes in WT mice, but not, of course, in TAAR1 KO mice, mirroring what was seen with MDMA on its own.[66] This study seems to suggest that TAAR1 agonism is not meaningfully involved in MRA induction of monoamine release in vivo and instead serves overall solely to inhibit it. Accordingly, the paper's title was "Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA)". I also looked up cocaine in TAAR1 KO mice, and cocaine-induced locomotor activity (and presumably by extension dopamine elevation) was no different between TAAR1 KO mice and WT mice, similarly to the case of PCA.[67] Hence, it seems that it is specifically MRAs(/MRIs) acting as TAAR1 agonists that are affected by TAAR1 KO, not MRAs in general, and TAAR1 agonism by these agents is self-inhibiting.
- User:Seppi333, feel free to chime in on any of these as well. – AlyInWikiWonderland (talk, contribs) 00:27, 16 December 2024 (UTC)
- No worries. Glad to hear my reply was helpful. :)
- Re: "
"TAAR1 is unequivocally a major intracellular target of amphetamine, [...]". I understand that you and User:Seppi333 feel this way. And you may be correct. But this is an uncited assertion. What are the hard sources/evidence to show that this is true?
" - The citations are in the wikitable I posted. The data indicates that PKC is responsible for ~50% of DA efflux. The data also indicates that TAAR1-mediated PKA and PKC signaling cascades are involved in amphetamine-induced DA reuptake inhibition (i.e., transporter internilisation). With regard to DA efflux specifically, amphetamine induces a PKC signaling cascade via TAAR1 and another yet unidentified mechanism that then induces a CAMKII-alpha signalling cascade, with the former account for 50% of DA efflux. This citation attributes a specific percentage of dopamine efflux to a protein kinase. "
Consistent with this idea, PKCb knockout mice demonstrate reduced, though notably not completely eliminated, AMPH-evoked DA efflux (Chen et al., 2009). Similar results were recently observed by the Gnegy group, who found that perfusion of PKCb inhibitors into the nucleus accumbens of rats reduced AMPHevoked DA efflux by approximately 50% (Zestos et al., 2016).
"[54] - Moreover, this review indicates: "
The fact that TAAR1 is a direct, high affinity target for METH and AMPH whereas the DA D2R is not (Xie et al., 2007b) supports the idea that METH/AMPH interferes with TAAR1/DA D2R functional and/or physical interaction(s). ... The interpretation made of these data is that as a TAAR1 ligand/DAT substrate (i.e. METH/AMPH/DA) enters the cell via the DAT and accumulates, TAAR1 signaling progressively occurs. In turn, phosphorylation cascades are promoted ultimately modulating DAT kinetics.
"[59] - Re: "
I feel like the relevant parts of these articles in general should start with something like "amphetamine appears to induce monoamine efflux via multiple known targets, including [...]". Right now, they start with TAAR1 agonism, and CAMKII is left almost as an afterthought.
" - I'm not opposed to this and I think Seppi333 could be on board for this because they actually intended to make this change a few years ago. For context, at the end of 2019 Seppi had expressed desire to update amphetamine's pharmacology section to reflect the current (at the time) literature covering amphetamine's pharmacodynamics in DA neurons. The wikitable I linked above was Seppi's work and this table was to be added to supplement an updated graphic (i.e., the amphetamine pharmacodyanmics in dopamine neurons template). However, that hasn't come about yet and TMK that's because of two reasons. (1) I believe Seppi was waiting for the CAMKII-alpha cascade to be fully elucidated and (2) Seppi became seldom active on Wikipedia shortly after because I'm assuming he was motivated to prioritise his time toward the development of his new company. Furthermore, over the years no other editor - besides myself - has expressed an interest in majorly overhauling the amphetamine article in a manner that's consistent with its featured article status. I would certainly like to see an update to the pharmacology section with that table alongside the redrawn diagram template. Seppi previously drafted the following sentence as an appended note to the wikitable and I think something analogous to that would improve the article.
- "
Amphetamine interacts with its receptor protein target(s) (i.e., TAAR1 and a currently unidentified biomolecular target which initiates its CAMKIIα cascade), which triggers the activation of protein kinases. The activated kinases then phosphorylate their respective transporter(s), which in turn causes a conformational change in transporter protein, thereby altering its function and affecting dopaminergic/glutamatergic neurotransmission at dopaminergic synapses.
" - I'm happy to work on updating/redrawing the SVG of the amphetamine pharmacodynamics template sometime over the next week/the holiday season. This would likely be based on the figure in this paper[55]; i.e., the RhoA signalling cascade would be drawn from TAAR1 and the CAMKII-alpha signalling cascade will likely involve appending an "Unidentified intracellular pathway" figure before drawing the pathway to DAT to illustrate that it confers DA efflux. I'll also have add glutamatergic pharmacodynamics of amphetamine in DA neurons (i.e., EAAT2 internalisation and VGLUT2 in the axon terminal). Professional Crastination (talk) 12:40, 16 December 2024 (UTC)
- User:Seppi333, feel free to chime in on any of these as well. – AlyInWikiWonderland (talk, contribs) 00:27, 16 December 2024 (UTC)
I appreciate you reposting that table Professional Crastination; I actually forgot about that.
Anyway, I'll address a few points: I really don't know or care about most structural analogs of amphetamine. Structure activity relationships only go so far, and drugs like cathinones have virtually nothing in common with amphetamine in a pharmacodynamic sense despite having amphetamine in its structural backbone. Even just N-methylating amphetamine changes some of its downstream targets from TAAR1 and the VMAT2 binding site; tacking more crap onto that backbone like a methylenedioxy group would only introduce further changes. But, monoamine-releasing agents are simply a drug class that shares a common mode of action, not a common chemical structure or a common mechanism of action that mediates said mode. That's why I don't care to comment on that point. I simply know close to nothing about most MRAs, which is a very large drug class.
Pharmacodynamics of amphetamine in a dopamine neuron
|
As for amphetamine and TAAR1, yes the relationship there is paradoxical. But the same is true for amphetamine and VMAT2. Amphetamine binds at the tetrabenazine binding site on the latter protein. What does tetrabenazine do, though? Well, it effectively sequesters most vesicular dopamine within the cytosol of a neuron, where it just gets broken down because it's not being pumped out through a transporter or dumped into the synapse within a vesicle. As for selective TAAR1 full agonists? Well sure, they induce reverse transport and reuptake inhibition at transporter proteins, but unless there's a ton of dopamine within the neuronal cytosol, that mechanism of action is not going to induce any notable monoamine release; they're not like amphetamine at all. Amphetamine's activity at TAAR1 and VMAT2 individually is actually strongly inhibitory in dopamine neurons, which is exemplified by drugs that selectively interact with those proteins; but, the combination of those two mechanisms strongly promotes monoamine release. There is very likely another intracellular target of amphetamine that mediates reverse transport at DAT, but it's not required for it to act as an MRA. Seppi333 (Insert 2¢) 19:20, 14 December 2024 (UTC)
- I would argue that you should care about other MRAs though. Because they're closely structurally related to amphetamine, surely have substantial overlap with the mechanisms of amphetamine, and can provide indirect insights into amphetamine's pharmacology. Personally I think the lack of hTAAR1 agonism with many of them should be telling in terms of weighing the likely relative significance of TAAR1 agonism versus other mechanisms to monoamine release induced by amphetamine. Does that not make you think for a moment too? Relatedly, you could also argue something similar about the importance of VMAT2 in monoamine release with amphetamine and other MRAs (see what I wrote about VMAT2 above).
- On this topic of other MRAs, I'd like to also note that this TAAR1 agonism theory of MRA monoamine release has been applied to other MRAs on Wikipedia, for instance at monoamine releasing agent, TAAR1, MDMA, phentermine, and propylhexedrine among others. I don't know if that was always you or others. And it might of course prove to be valid in some cases. But obviously applying TAAR1 agonism as the mechanism of monoamine release to MRAs in general needs to be considered with significant nuance. I do not think it's likely to apply to MDMA for instance and that content should be corrected.
- I should note that I'm not mystified by the fact that selective TAAR1 agonists don't have amphetamine-like effects. The need for concomitant MAT uptake (and, per you, VMAT2 activity) aspect of things has made sense to me there. While amphetamine (and other sufficiently lipophilic TAAR1 agonists) can passively diffuse into cells, active transport by MATs appears to dramatically increase its passage.[48] This, in turn, will result in a very different pattern or distribution of TAAR1 signaling in the CNS, and by extension the potential for very different effects. At least on a theoretical level and assuming the TAAR1 MRA theory is true. It's more all of the other points I mentioned that have given me pause.
- Besides the other things I mentioned above, I'm also still quite concerned about the fact that the amphetamine TAAR1 MRA theory appears to be very obscure and not well-established or widely accepted. Looking to get some more insight, I emailed Dr. David Sulzer at Columbia University today. He is the originator of the "weak base theory", is one of the world's leading experts on amphetamine pharmacology (or perhaps the leading expert, per Hamilton Morris),[74] and is the lead author of a major 2005 review on mechanisms of amphetamine-induced monoamine release.[75] He had never heard of TAAR1 mediating amphetamine-induced monoamine release before. I showed him the Miller and Xie studies and asked him what he thought about them and the theory. He said they were interesting. But he looked up TAAR1 KO studies by his own accord and discounted the theory. He said that you should read more of the well-accepted literature on this topic and provided two of his reviews, one of which was the 2005 review (which he said was mostly accurate but outdated due to lacking Paul Phillip's more recent findings).[75][76] Along similar lines, a very relevant 2024 review, "Post-Translational Mechanisms in Psychostimulant-Induced Neurotransmitter Efflux", barely mentions the TAAR1.[77] Same again for a 2020 review by Maarten Reith (another well-known expert on MRAs) and colleagues, "Molecular Mechanisms of Amphetamines"—it implicated DAT, VMAT2, and OCT3, as well as PKC, CaMKII, and ERK, but no mention of TAAR1 at all.[48] These are major recent literature reviews on amphetamine mechanisms and yet they barely mention TAAR1, which you assert is the key mediator of amphetamine actions.
- I've only implied this before, but Wikipedia policy dictates that content should, of course, reflect mainstream scientific views and consensus:
- "In Wikipedia parlance, the term fringe theory is used in a broad sense to describe an idea that departs significantly from the prevailing views or mainstream views in its particular field. Because Wikipedia aims to summarize significant opinions with representation in proportion to their prominence, a Wikipedia article should not make a fringe theory appear more notable or more widely accepted than it is. Statements about the truth of a theory must be based upon independent reliable sources. If discussed in an article about a mainstream idea, a theory that is not broadly supported by scholarship in its field must not be given undue weight,[1] and reliable sources must be cited that affirm the relationship of the marginal idea to the mainstream idea in a serious and substantial manner." (WP:FRINGE)
- It does not currently appear to be scientific consensus, let alone even well-known, that TAAR1 agonism mediates MRA actions of amphetamine and that this is relevant in humans. It's an obscure and limitedly supported theory. And a theoretical possibility that indeed might have merit, sure. But clearly more research seems necessary to demonstrate that it has basis. Miller himself, the originator of the idea, has shown due restraint in this regard in his publications, as would be expected of any rigorous scientist; from a 2016 review he coauthored: "That TAAR1 signaling is coupled to the inhibition of VTA DA neuron firing was a surprising finding. [...] Nonetheless, this mismatch in expectations immediately attracted the attention of those trying to determine whether TAAR1 is a METH/AMPH and DA receptor in vivo as it is in vitro (Bunzow et al., 2001; Wainscott et al., 2007; Xie and Miller, 2009; Panas et al., 2012)."[65] I'm currently conversing with Miller by email and getting his take on things as well. He has expressed that these in-vitro findings are highly methodology-dependent and you can potentially obtain even opposite results with different in-vitro systems and techniques (due, e.g. and among other factors, to TAAR1-mediated DAT internalization being inhibitory not only of reuptake but also of efflux). In turn, he has placed a great deal of value on in-vivo research, for instance with KO mice as well as canines (which notably lack a functional TAAR1[78]).
- Based on all of this, I think that the current content on Wikipedia re: TAAR1 agonism being the mediator of the monoamine release of amphetamine and other MRAs needs to be presented much more conservatively. Because right now people reading it will just assume that it's definitively how amphetamine and other MRAs work, and that is not currently what the literature reflects. I think the content should say something like "The mechanisms of amphetamine-/MRA-induced monoamine release are unknown. [...] TAAR1 agonism (also CAMKII) is one possible contributing factor based on in-vitro studies. In any case, more research is needed." (Not verbatim. Just a very rough idea or start.) That kind of approach would be much more true to the current scientific literature. – AlyInWikiWonderland (talk, contribs) 00:27, 16 December 2024 (UTC)
- TAAR1 agonism is a mechanism of action for any MRA even for selective full agonists; it's just not that notable as I said before. It is well established in every review and primary source that's been cited. Is it the primary mechanism of action? Perhaps not for some drugs like phentermine, which isn't really a releasing agent at all. But, until you can substantiate your theory that TAAR1-mediated PKCβ-induced DAT reverse transport insignificantly affects DA efflux relative to CAMKIIα, this is a moot point to argue given that there is no evidence to support that assertion. Seppi333 (Insert 2¢) 10:35, 16 December 2024 (UTC)
- But hey, if you really want to test it in vivo, buy a selective full hTAAR1 agonist and tetrabenazine from sigma aldrich and take them both, then you'll know whether you're right. Seppi333 (Insert 2¢) 11:01, 16 December 2024 (UTC)
- I have to go for now, but I wanted to leave something here before I do. Another study recently attempted to replicate Xie & Miller's findings on methamphetamine in vitro and failed to do so:[79]
- "Previous research on TAAR1 modulation of DAT function has produced equivocal findings. In vitro, MA inhibits [3H]DA uptake, and [3H]DA release is increased in striatal tissue from Taar1 WT compared with KO mice (Xie and Miller, 2009). Similar findings were described in cells cotransfected with TAAR1 and DAT, compared with cells transfected only with DAT, in which MA-induced [3H]DA uptake inhibition and release were increased (Xie and Miller, 2007, 2009). However, these findings indicate MA-induced impairment of DAT function is increased when TAAR1 is activated, as opposed to in vivo treatment with AMPH or MDMA, by which striatal extracellular DA levels are increased when TAAR1 is not activated (Wolinsky et al., 2007; Lindemann et al., 2008; Di Cara et al., 2011). We were unable to replicate the results of Xie and Miller (2009) under similar in vitro conditions (Fig. 3). There was no difference in IC50 values for [3H]DA uptake inhibition by MA between synaptosomes from Taar1 WT and KO mice. [...]
- [...] our results do not support an earlier hypothesis that TAAR1 modulates DAT (Xie and Miller, 2007, 2009; Xie et al., 2008b), as there was no evidence of an interaction under conditions described above. Recent reports support our findings that the DAT is unaffected by TAAR1. Coadministration of MA and the TAAR1 partial agonist RO523648 did not alter [3H]DA uptake and release in striatal synaptosomes in rats (Cotter et al., 2015). Fast-scan cyclic voltammetry showed no difference in DA clearance, as mediated by DAT, in striatal tissue from Taar1 WT compared with KO mice (Leo et al., 2014). Finally, selective TAAR1 agonists diminished hyperlocomotion in DAT KO mice and DAT KO rats, providing behavioral evidence that TAAR1 signals independently of DAT (Revel et al., 2011; Leo et al., 2018). Given the lack of interaction, DAT is an improbable mediator of TAAR1 regulation of MA-induced neurotoxicity.
- [...] activation of TAAR1 did not modulate in vitro MA-impairment of DAT function or DAT expression. As TAAR1 activation did not alter the function or expression of DAT in whole synaptosomes or VMAT2 located on membrane-associated vesicles, these results indicate TAAR1 does not interact with these transporters on the plasma membrane but does affect intracellular VMAT2 function."
- I really don't know how you could continue to assert this theory with so much counterevidence. I have also reached out to Dr. Maarten Reith and asked him his opinions about the TAAR1 agonism theory. We'll see what he says as well. I suspect he did not include the TAAR1 agonism theory in his 2020 review because there are so many findings that overtly contradict it (particularly the TAAR1 KO studies). At this point, I really think you need to concede that your theory is highly uncertain, that it is not scientific consensus or widely accepted, and that the current content on Wikipedia needs to be refactored.
- I'll respond to the rest of the comments here later. – AlyInWikiWonderland (talk, contribs) 06:06, 17 December 2024 (UTC)
References
[edit]References
|
|---|
|
References
|
Discussion to rename article title from Niacin to Nicotinic acid
[edit]This is an invitation to join the discussion at Talk:Niacin#Requested move 15 December 2024
Thanks! -- Arthurfragoso (talk) 00:42, 16 December 2024 (UTC)