Talk:Sulfur dioxide
 | This article is written in American English with IUPAC spelling (color, defense, traveled; aluminium, sulfur and caesium) and some terms that are used in it may be different or absent from other varieties of English. According to the relevant style guide and chemistry naming conventions, this should not be changed without broad consensus. |
| Discussion of spelling archived to Talk:Sulfur/Spelling. Take note of WP:SULF. |
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
Dead Link
[edit]The link to IARC monographs v54, containing the article "Sulfur Dioxide and some Sulfites, Bisulfites and Metabisulfites" was broken (apparently by a reorganization of the IARC website).
The old link went to: http://www-cie.iarc.fr/htdocs/monographs/vol54/02-sulfur-dioxide.htm
I found the monograph pdf at: http://monographs.iarc.fr/ENG/Monographs/vol54/volume54.pdf —Preceding unsigned comment added by 71.206.233.111 (talk) 02:43, 22 March 2009 (UTC)
STRUCTURE
[edit]It has been brought to my attention that the structure put here is entirely WRONG, there is resonance in the sulfur dioxide molecule between O-S=O, and O=S-O, if anything one of these structures should be posted, O=S=O is wrong entirely. -Annoyed Chem Student —Preceding unsigned comment added by 71.145.153.177 (talk) 21:57, 3 September 2007 (UTC) Dear annoyed - most chemical structures are wrong in some sense, but not entirely and certainly with no bad intentions. Chemists specify "structures' that are highly simplified relative to the quantum reality that is undescribable with letters and lines. We also seek conciseness and, by agreement, draw one of many resonance structures. --Smokefoot 22:47, 3 September 2007 (UTC)
Career and PhD chemists with years of experience may not need Wikipedia to specify different forms of SO2, but neophytes who are our most likely readers are looking for basic and comprehensive details. Current teaching states that there are 18 valence electrons in SO2. The structure O=S=O requires only 16 electrons. O-S=O and O=S-O require 18, and the uncertainty of the location of the double bond seems to me to be entirely in keeping with the quantum mechanics involved. IMHO O=S=O should not be displayed as the sole structure, and if it is displayed at all, there should be an explanation that the rest of the world can understand. O-S=O and/or O=S-O should be displayed as well, with footnotes as appropriate. ESROB —Preceding comment was added at 00:36, 20 November 2007 (UTC)
- See below. O=S=O has 18 valence electrons, just like O=S+−O− and −O−S+=O.
 |
Ben (talk) 16:53, 4 May 2008 (UTC)
- I just caught one of my college level chemistry students in an error thanks to this page. The current model appears to only have 16 valence electrons: 2 lone pairs on each of two oxygens for a total of 8 electrons, and 2 double bonds for a total of 8 electrons. Grand total: 16 valence electrons that are apparent to those less experienced with chemistry, not 18 (the correct number for the molecule). I had to explain to my student what was wrong, and why the diagram seems wrong. Regardless of what various professors think is the best way to represent the structure, or what old chemistry books say, the current structure is causing students to make mistakes. Can we please get a diagram that addresses this problem? ----Pich (Pich) 14:39, 3 February 2009 (UTC)
If we draw all the lone pairs (including on sulfur) there should be no ambiguity and it should be clear that this is an 18-valence-electron molecule. Here's the image:
Ben (talk) 20:30, 3 February 2009 (UTC)
Ben is reight, but not quite exactly because it has a resonance structure not shown in his picture. You should be sure to address the other possible structure, because in reality, it is an average of the two structures. -Chem student — Preceding unsigned comment added by 75.90.34.4 (talk) 22:02, 3 August 2014 (UTC)
I came here as a newbie to chemistry, and was indeed very confused by the two double bindings. The problem with the O=S=O structure, is that S will apparently have ten electrons in outermost shell, when the two free electrons are added. I think this should be updated to show the one double and one single binding instead, as it's more "correct" from a newbie point of view (which will be the more common audience on a wiki article about this compound). 178.232.0.180 (talk) 13:50, 4 September 2018 (UTC)
Many sources have the structure as O=S=O with an unshared pair on the sulfur atom, making the total number of valence electrons the correct 18, and making it a bent molecule, with no resonance. If there is no definitive source, both of these models should be presented as possibilities. The one with the two double bonds ostensibly is more likely as all formal charges are zero, albeit with the sulfur having 10 valence electrons, which is quite common for sulfur. 50.224.111.240 (talk) 13:40, 27 November 2024 (UTC)
Picture
[edit]Can we get a better picture?? -Hamdev Guru 16:04, 3 October 2005 (UTC)
Sulphur
[edit]Sulphur is spelt with a ph, not an f, as it comes from English, not American. —The preceding unsigned comment was added by 90.197.207.136 (talk • contribs) 17:57, 3 December 2006 (UTC).
- See the appropriate style guidelines. E.g. wp:chem. --Dirk Beetstra T C 18:03, 3 December 2006 (UTC)
I'll drink to that. SulFur is just promoting laziness.
AND BY THE WAY... (to you sulFur fellas) AluminIUm sounds the same way it's spelt, funnily enough!
al-loo-min-ee-um
NOT
al-loo-min-um
- —Preceding unsigned comment added by 62.31.155.153 (talk • contribs) 21:58, 14 March 2007
- -It is not about laziness, 'sulfur' is recognised as international name for element with atomic number 16 of now days Kboom 09:44, 9 May 2007 (UTC)
sulfur
[edit]is sulfur dioxide an organic vapor?
- It is a vapor, but not an organic one as it is not carbon-based. Walkerma 01:51, 9 May 2006 (UTC)
What are the reccmommended Protective equipment which should be used in the industry? please explain
sulfur forms the compound S8.
Emissions section misinforms
[edit]The Emissions section as written in fall, 2007, is misleading as to the extent and causes of U.S. sulfur dioxide emissions reductions and frankly sounds like it was written by an energy industry apologist. From 1970 through 2005, U.S. EPA 5-year and 10-year data points show total annual emissions falling from 31.16 to 14.63 million tons in nearly a straight line, for a reduction of more than half. The great majority of this long-term trend has been achieved by fuel substitution, chiefly low-sulfur coal and diesel, not by flue gas scrubbing. —Preceding unsigned comment added by 151.199.44.181 (talk) 12:08, 13 October 2007 (UTC) :Wikipedia exists to be edited by people like you. So you are welcome to fix the problem.--Smokefoot 14:27, 13 October 2007 (UTC)
Structural picture
[edit]It would be nice if the molecular diagram was bent to show the actual geometry of the bonds. I was temporarily confused, thinking the current picture implies it was linear. -postglock 12:25, 25 April 2006 (UTC)
Also the bond between 1 of the oxygens and the sulfur is a coordinate covalent bond which would make it a bend single bond between 1 of the oxygens and the sulfur. —The preceding unsigned comment was added by Hunter839 (talk • contribs) .
- I'll try and fix this picture. Walkerma 01:51, 9 May 2006 (UTC)
Picture
[edit]The image at the top right of the page showing the connections between the sulfur and the 2 oxygen is inccorect. There is only one double bond but the picture shows two. If there was 2 double bonds the sulfur would have 10 electrons in its outer layer. The picture should only have one double bond with a picture looking similar to this O=S-O. unsigned by User:Lambgina
- Here is the key issue to ponder and read about: Why are you so certain that "there is only one double bond?" and that something is wrong about "10 electrons"? How do you propose to describe the bonding in DMSO, sulfuric acid, [[SF6]]?--Smokefoot 11:41, 25 October 2006 (UTC)
- You could always start with our short article on hypervalent molecules... Physchim62 (talk) 11:56, 25 October 2006 (UTC)
Well thank you now I understand and my goals in life have been completed. I can now die satisfied with my vegemite sandwich.
The appendix section
[edit]Why I see the Appendix: temperature dependence of aqueous solubility looks so confused and I could hardly identify the information due to its mixed-up in format style. Can anyon (Talk) 14:14, 2 March 2007 (UTC)
Can I say on the picture (3D digram), sulfur dioxide has a bent structure with the sulfur atom having two free electrons (a lone pair).22 May 2007—Preceding unsigned comment added by 61.9.198.179 (talk • contribs)
- This should not go in the picture itself. However, if you want to add to the section Sulfur_dioxide#Structure_and_bonding that would be good. Walkerma 05:30, 22 May 2007 (UTC)
sulphur dioxide as a preservative
[edit]I quite often find this in various food products and am aware of what it's used for but is it safe to ingest?203.221.26.195 12:19, 22 March 2007 (UTC)
Sulfur Dioxide can be very poisonous, but -as is the case with nearly all preservatives- it is used in VERY small quantities (too small to be dangerous). There have been objections to its use in the past, but it is relatively benign. I've actually had to research its use as a preservative before, and I didn't find any dangers with its consumption as a preservative. --Xeraxes (talk) 01:16, 20 January 2009 (UTC)
I'm very new at this, but could not find a more appropriate place to post this. Apologies if this is in error. Any advice would be appreciated.
I was alarmed by the misinformation that appeared under Uses 3.3 Wine. Here's the quote:
"SO2 is also a very important element in winery sanitation. Wineries and equipment must be kept clean, and because bleach cannot be used in a winery, a mixture of SO2, water, and citric acid is commonly used to clean and sanitize equipment. Compounds of ozone (O3) are now used extensively as cleaning products in wineries due to their efficiency, and because these compounds do not affect the wine or equipment."
I was a wine-maker for a number of years, and the above quote is either incorrect or misunderstood when reading source material. Stainless steel tanks, pumps,hoses etc., are normally cleaned with a circulating cleanser which is mostly sodium hydroxide (lye). A water rinse is followed by recirculated citric acid to neutralize any residue of the quite basic initial cleanser. Finally, chlorine at 100 ppm is recirculated and allowed to air dry. Chlorine bleach is by far the main sanitizer used by wineries. SO2 is never used on stainless fermenters because wine yeast is so easily killed by it.
Part of the description I'm disputing, seems to be referring to the procedure used to clean and prepare wine barrels for a period of empty storage. This is the only time SO2 is used in a fermenting vessel--usually in gaseous form--because the next time juice is added to ferment, any free sulfur will be long gone.
One of the main reasons SO2 is not used near live, active yeast cultures, is wine-makers don't want their yeast strains to begin to develop resistance to it's effects. Not unlike the over-use of human antibiotics.MigcaX (talk) 09:32, 11 January 2010 (UTC)
Comment from article re: structural image
[edit]Removed the following from article top:
- the picture for this article is incorrect. SO2 has a resonance structure and isn't permanently bonded. It looks more like NO2-. Sorry I posted this here, I don't know how to change the picture on the right column. Source: http://www.cartage.org.lb/en/themes/sciences/chemistry/Inorganicchemistry/Informationbonding/bondingindex/Resonance/Resonance.htm
Added by —Preceding unsigned comment added by 70.187.239.252 (talk • contribs) 23:32, 22 March 2007
- Vsmith 00:17, 23 March 2007 (UTC)
- The structure looks fine to me - it shows one of the three valid resonance forms for the compound, the other two being the zwitterionic ones given in the cited ref. Unlike N in NO2−, S can expand its octet and have two double bonds while being neutral. Walkerma 06:38, 23 March 2007 (UTC)
fungicide
[edit]My "Subsole" brand grapes say that they have been "treated with sulfur dioxide for fungicide use." Does anyone have any information on this, to add to the "uses" section? I want to know if it's safe to eat.
becky
MSDS
[edit]Why is there no MSDS on this page? —Preceding unsigned comment added by 63.3.16.1 (talk • contribs) 16 Aug 2007
Error?
[edit]Different inorganic chemistry books say different things. The one I was looking at first said that sulfur dioxide resonates between O-S=O and O=S-O only. Another one says that only O=S=O is the correct representation. Ben: if you could post the two resonance structures in addition to the structure currently posted and add an explanation, that would be most accurate and informative to readers of the article.
- Done.
- Ben (talk) 21:19, 12 April 2008 (UTC)
- See also hypervalent molecule: the two descriptions are actually equivalent, but some chemists prefer one and some chemists the other. Physchim62 (talk) 11:44, 13 April 2008 (UTC)
SO2 is often described as the "smell of burning sulfur"
[edit]ORLY? Surely this is a mockery. Of course a sulfur oxide smells like sulfur oxidising. —Preceding unsigned comment added by 123.2.55.229 (talk) 05:53, 24 April 2008 (UTC)
== I think the gas which has the smell described in the header is Hydrogen Sulphide, not Sulphur Dioxide. Psic88 (talk) 01:24, 19 April 2014 (UTC)
WikiProject Food and drink Tagging
[edit]This article talk page was automatically added with {{WikiProject Food and drink}} banner as it falls under Category:Food or one of its subcategories. If you find this addition an error, Kindly undo the changes and update the inappropriate categories if needed. The bot was instructed to tagg these articles upon consenus from WikiProject Food and drink. You can find the related request for tagging here . Maximum and careful attention was done to avoid any wrongly tagging any categories , but mistakes may happen... If you have concerns , please inform on the project talk page -- TinucherianBot (talk) 01:25, 4 July 2008 (UTC)
Largest Producer?
[edit]I'm currently looking for the world's largest supplier of Sulfur Dioxide (as a preservative or general) but I have had no luck at all finding one. This could be because many of the companies produce their own SO2, but there still has to be some supplier. I'm stating this because there are no major suppliers listed here... Anyone care to help? --72.137.83.62 (talk) 02:17, 6 January 2009 (UTC)
- The people who sell it will be the major industrial gas suppliers – Linde Group, Air Liquide, Praxair and Air Products. They probably don't make it themselves (although they may well purify it, especially for food use) but but it in from the manufacturers of sulfuric acid, for which read virtually any major chemical company. Physchim62 (talk) 02:39, 6 January 2009 (UTC)
Allergen?
[edit]Why is no allergey information listed for this?
Or the fact that it is a common allergen should at least be listed.
http://www.food.gov.uk/safereating/allergyintol/alerts/2008/dec/apricotchutneysupdate —Preceding unsigned comment added by Wamatt (talk • contribs) 12:58, 1 February 2009 (UTC)
Sulphur dioxide, sodium benzoate and tartrazine are well known culprits that cause wheeziness and ‘tight chests’ in asthmatics. Other people develop sore, scratchy throats or skin rashes and hives. http://health.mweb.co.za/dietnfood/Allergy_intolerance/15-3110-3112-3129,14452.asp —Preceding unsigned comment added by Wamatt (talk • contribs) 13:00, 1 February 2009 (UTC)
Sorry but I removed the use of the word allergy from the safety section. The pages on allergen and antigen indicate that true allergens are a narrower group of more complex biomolecules. The safety section could be beefed up, but words like toxicity, sensitivity, or intolerance would probably be more accurate. 99.175.103.243 (talk) —Preceding undated comment added 10:34, 4 September 2009 (UTC).
- Suphite sensitivity is a well established, major health concern: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017440/
bond order of at least 2?
[edit]Depending on the contribution of each resonant structure, the bond order should lie somewhere between 1 and 2. (If we assume equal contribution, then it's 1.67) How can SO2 have a bond order of at least 2? 202.40.137.197 (talk) 10:49, 10 January 2010 (UTC)
Uses 3.3 Wine
[edit]There appears to be some misunderstanding or misinformation regarding the use of sulfur dioxide in commercial wine production.
Here's the quote:
"SO2 is also a very important element in winery sanitation. Wineries and equipment must be kept clean, and because bleach cannot be used in a winery, a mixture of SO2, water, and citric acid is commonly used to clean and sanitize equipment. Compounds of ozone (O3) are now used extensively as cleaning products in wineries due to their efficiency, and because these compounds do not affect the wine or equipment."
I was a wine-maker for a number of years, and the above quote is simply incorrect or is a misunderstanding of the source material (none is cited).
The usual method for cleaning and sanitizing stainless steel tanks, pumps, hoses, fittings, etc., starts with a pump-circulated cleanser that consists mostly of sodium hydroxide (lye). A water rinse is followed by recirculated citric acid to neutralize any residue of the initial cleanser, which is a strong base. After another rinse, a solution of 100 ppm of chlorine is briefly recirculated, drained, and then allowed to air dry.
Chlorine bleach is by far the primary sanitizer used in wineries. SO2 is never used on stainless fermenters because wine yeast is so easily killed by it.
Part of the description I'm disputing, seems to be referring to a portion of the procedure used to clean and prepare wine barrels for a period of empty storage. This is the only time SO2 is used as a sanitizer in a fermenting vessel--usually in gaseous form--this works because the next time juice is added to barrel ferment, any free sulfur will be long gone.
Another reason SO2 is not used near live, active yeast cultures, is wine-makers do not want their yeast strains to begin to develop resistance to it's effects. This is very similar to the resistant strains of disease-causing bacteria due to the over-use of human antibiotics.--MigcaX (talk) 06:58, 12 January 2010 (UTC)
- Sulfur dioxide. What is it? An acid or a base. 120.60.10.39 (talk) 11:18, 28 March 2010 (UTC)
- I think the right answer is that sulfurous acid is in equilibrium with water and sulfur dioxide. But sulfur dioxide in a dry environment is a reducing agent. --Chris.urs-o (talk) 11:29, 28 March 2010 (UTC)
Source of Global Warming
[edit]Apparently there is some evidence that SO2 is the main driver for global warming. The full details still need to be worked out. See one of the first papers: Ward, P. L. (2009). "Sulfur dioxide initiates global climate change in four ways". Thin Solid Films. 517: 3188. doi:10.1016/j.tsf.2009.01.005.
- Ward, Peter L. (2 April 2009). "Sulfur Dioxide Initiates Global Climate Change in Four Ways" (PDF). Thin Solid Films. 517 (11): 3188–3203. doi:10.1016/j.tsf.2009.01.005. Retrieved 2010-03-19. --Chris.urs-o (talk) 18:40, 21 April 2010 (UTC)
Listing of amount of SO2 by the U.S. change
[edit]Hello, I made a change to the order of the listing of the amounts of SO2 under the section "As an air pollutant". It was, before the change, listed as thus:
* 1999 18,867 * 1998 19,491 * 1997 19,363 * 1996 18,859 * 1990 23,678 * 1980 25,905 * 1970 31,161
I changed it to this:
* 1970 31,161 * 1980 25,905 * 1990 23,678 * 1996 18,859 * 1997 19,363 * 1998 19,491 * 1999 18,867
So, I reversed the order so the oldest date would read first and progress from there, instead of the other way around. I did this because, as far as I can tell, historical listing of dates starts at the oldest and progresses to the newest in English, as we read left to right, top to bottom. As the old way, it can be confusing as one might think over time the amounts were actually increasing over time, instead of decreasing if not observed carefully. I myself read it first as it was increasing over time, but after looking it over again I saw it was in fact decreasing, he cause of this human error being the way it was listed. I actually looked at the source of the listing itself http://www.epa.gov/air/airtrends/sulfur.html to make sure that perhaps it was not just an editor's error in writing the dates when it implied that it was decreasing over time, but saw it was the truth!
Sorry for all this talking over this little change, if you find it was not a good decision, please tell me here in the discussion and we can talk about it.
Thank you for your time! — Preceding unsigned comment added by 75.73.114.111 (talk) 18:39, 25 June 2011 (UTC)
Reducing Agent
[edit]Sulfur dioxide is purchased in quantity for use as a reducing agent. It is accurate that the active reductant is the hydrogen sulfite ion, HSO3, a large amount of industrial reduction is done by pumping sulfur dioxide into an aqueous solution of whatever it is that needs to be reduced. When dissolved in water, sulfur dioxide produces a concentration of hydrogen sulfite ion, ostensibly from the hypothetical sulfurous acid. IR spectrography of an aqueous solution of sulfur dioxide shows only spectra of sulfur dioxide, and none of sulfite ion.
Theoretical considerations to the contrary notwithstanding, sulfur dioxide is used in quantity as a reducing agent, and to state that it is not such is solopism. Norm Reitzel (talk) 22:00, 19 February 2012 (UTC)
- You are asking for comment not the claim that SO2 is a reducing agent when the active agent is actually HSO3-. It seems that this phenomenon might be useful to explain in the article.
- I guess that we claim that many compounds undergo hydrolysis implying a role for water, when the active agent might be OH-. So ambiguity often is associated with a reagent being active or a precursor. This kind of semantic issue comes up often in catalysis - is Wilkinson's catalyst (RhCl(PPh3)3) a hydrogenation catalyst or is it a pre-catalyst? I hope I understand your question well.--Smokefoot (talk) 04:25, 20 February 2012 (UTC)
- Actually, that is exactly the guidance I was looking for. I did not think it appropriate to remove mention of sulfur dioxide as a reducing agent since it is bought in quantity for exactly that reason. I'll explain in the article in some detail how the reduction takes place. Norm Reitzel (talk) 05:02, 21 February 2012 (UTC)
corrected bonding section
[edit]The previous information contrasting the bonds in sulfur dioxide with ozone, in that the S–O bond in sulfur dioxide being shorter than that of sulfur monoxide, but O–O bond in ozone longer than oxygen, is incorrect as the information was comparing sulfur dioxide with the diradical sulfur monoxide, yet compared ozone with singlet oxygen rather than (diradical) triplet oxygen as it should (which of course ozone has a shorter bond length comparatively). Hence, both sulfur dioxide and ozone actually have equivalent bonding and I have changed the information to instead reflect 3c-4e bonding as normally used to explain "hypervalent" molecules.--Officer781 (talk) 16:15, 4 September 2012 (UTC)
- Good catch.--Smokefoot (talk) 22:19, 4 September 2012 (UTC)
- I have removed the reference to Greenwood and Earnshaw. The new information seems correct but it requires a reference, G&E reference was relevent to the information prior to change.Axiosaurus (talk) 10:00, 17 March 2013 (UTC)
I added the Greenwood & Earnshaw information. I'm not an expert in this area, but at least I paraphrased a reliable source and referenced it. What we have now is unreferenced, which is needs fixing ASAP. See WP:OR and WP:SYN. Officer781, where did you find this information? --Ben (talk) 15:58, 17 March 2013 (UTC)
- I have reread greenwoods account. The bond lengths according to NNG of O2, O3, SO and SO2 are O2 ground state 120.7 pm, singlets 121.55 pm, 122.77 pm (pp606), O3 127.8 pm, SO, 148.1 pm, SO2 143.1pm. So isn't Greenwood correct? The bond in SO is longer than that in SO2, whereas the bond in O2 is shorter (interestingly both for singlets and triplet forms) than that in O3. The O-O bond in O3 is NOT shorter than that of O2 as I think is being suggested above by Officer781, unless these numbers are incorrect or I am misinterpreting them or what officer781 is saying. Axiosaurus (talk) 11:59, 30 March 2013 (UTC)
- Hmm. The O-O bond in O3 is indeed longer due to the Lewis structure analysis of O2 having a bond order of 2 in each bond and O3 having a bond order of 1.5 instead. The SO bond is longer due to it being similar to a single bond (for some reason. See the sulfur monoxide page. It says that the bond length is similar to lower sulfur oxides having single bonds). I'll find the reference for the unreferenced information.--Officer781 (talk) 15:50, 3 April 2013 (UTC)
- But you're right on the triplet/singlet issue. I apologize for that mistake.--Officer781 (talk) 16:20, 3 April 2013 (UTC)
- Hmm. The O-O bond in O3 is indeed longer due to the Lewis structure analysis of O2 having a bond order of 2 in each bond and O3 having a bond order of 1.5 instead. The SO bond is longer due to it being similar to a single bond (for some reason. See the sulfur monoxide page. It says that the bond length is similar to lower sulfur oxides having single bonds). I'll find the reference for the unreferenced information.--Officer781 (talk) 15:50, 3 April 2013 (UTC)
Misbehaving images
[edit]These belong in the "Structure and bonding" section, but they don't seem to turn out right.
Perhaps someone can fix them.

--Rifleman 82 (talk) 17:38, 16 August 2013 (UTC)
Precursor to Particulates?
[edit]The article presently states that SO2 is a precursor to fine particulate soot. How does that work? Doesn't soot come from carbon compounds? Googling this just leads to lots of sites which just make the same statement with no support - many citing this article as their source. Sbreheny (talk) 08:43, 11 December 2013 (UTC)
- No mention of "soot" found in articles in March 2017. SO2 is a precursor to sulfate fine particulates, but not soot.
- When sulfur dioxide reacts with other chemicals in the air to form tiny sulfate particles, these particles can gather in the lungs and cause increased respiratory problems and difficulty breathing. Long-term exposure to sulfate particles can cause respiratory disease and even premature death.Feb 1, 2017
- Tox Town - Sulfur Dioxide - Toxic chemicals and environmental health ...
VB?
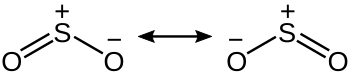
[edit]A recent edit (12/2/2014) which I undid as the charges were incorrect, highlights an issue. The verbal description reads like "classic" VB resonance (i.e. Lewis dots and octets) but lacks a picture to show the two canonicals, e.g O=S+–O- <-> -O–-+S=O which is unhelpful in my view. Also the reference is to a modern VB paper which sort of agrees with this description as it "confirms" the non-involvement of d orbitals. Any one out there with the necessary skills to put up a picture of Lewis octet resonance ? A full wikipedia treatment would also require some discussion of alternative views of the bonding. Axiosaurus (talk) 10:20, 12 February 2014 (UTC)
- Some people get agitated over this: but at least in the US, my feeling is that we virtually only describe molecules like SO2 in the MO context, at least at a college level. --Smokefoot (talk) 14:33, 12 February 2014 (UTC)
- As someone teaching that at a US university I have to disagree. We first discuss the Lewis/resonance picture, then clarify its inadequate nature, then describe molecules in terms of MO's. I personally skip the extended octet description, mostly because I think it is more fruitful for a gen chem student to spend some more time on MO's, then on trying to extend the Lewis story that I have just argued is not very adequate. CH 101 students tend to find MO's pretty challenging.
Jcwf (talk) 05:23, 25 February 2016 (UTC)
Canadian OHS fact sheet warns very toxic, with death or permanent severe disability possible from high or chronic exposure.
[edit]http://www.ccohs.ca/oshanswers/chemicals/chem_profiles/sulfurdi.html
- What are the potential health effects of sulfur dioxide?
Main Routes of Exposure: Inhalation.
Inhalation: VERY TOXIC, can cause death. Can cause severe irritation of the nose and throat. At high concentrations: can cause life-threatening accumulation of fluid in the lungs (pulmonary edema). Symptoms may include coughing, shortness of breath, difficult breathing and tightness in the chest. A single exposure to a high concentration can cause a long-lasting condition like asthma. If this occurs, many things like other chemicals or cold temperatures can easily irritate the airways. Symptoms may include shortness of breath, tightness in the chest and wheezing. {Reactive Airways Dysfunction Syndrome (RADS)}.
Skin Contact: CORROSIVE. The gas irritates or burns the skin. Permanent scarring can result. Direct contact with the liquefied gas can chill or freeze the skin (frostbite). Symptoms of mild frostbite include numbness, prickling and itching. Symptoms of more severe frostbite include a burning sensation and stiffness. The skin may become waxy white or yellow. Blistering, tissue death and infection may develop in severe cases.
Eye Contact: CORROSIVE. The gas irritates or burns the eyes. Permanent damage including blindness can result. Direct contact with the liquefied gas can freeze the eye. Permanent eye damage or blindness can result.
Ingestion: Not a relevant route of exposure (gas).
Effects of Long-Term (Chronic) Exposure: May harm the respiratory system. Can irritate and inflame the airways.
Carcinogenicity: Not known to cause cancer.
International Agency for Research on Cancer (IARC): Group 3 - Not classifiable as to its carcinogenicity to humans. American Conference for Governmental Industrial Hygienists (ACGIH): A4 - Not classifiable as a human carcinogen.
Teratogenicity / Embryotoxicity: Not known to harm the unborn child. Reproductive Toxicity: Not known to be a reproductive hazard. Mutagenicity: Suspect mutagen. May cause genetic damage based on animal information.
Ocdcntx (talk) 17:35, 26 March 2017 (UTC)
- Wikipedia is not in the advice and warning business. And Wikipedia-chemistry is mainly focused on, well, chemistry. As you have discovered, lots of other sites provide ample and excellent information on chemical hazards, real or exagerated (we smell SO2 everytime we light a match).--Smokefoot (talk) 18:48, 26 March 2017 (UTC)
External links modified
[edit]Hello fellow Wikipedians,
I have just modified 3 external links on Sulfur dioxide. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20130928111625/http://www.practicalwinery.com/janfeb09/page1.htm to http://www.practicalwinery.com/janfeb09/page1.htm
- Added archive https://web.archive.org/web/20130426082343/http://benthamscience.com/mrmc/contabs/mrmc10-11.html to http://benthamscience.com/mrmc/contabs/mrmc10-11.html
- Added archive https://web.archive.org/web/20090325155828/http://monographs.iarc.fr/ENG/Monographs/vol54/volume54.pdf to http://monographs.iarc.fr/ENG/Monographs/vol54/volume54.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 12:28, 5 December 2017 (UTC)
External links modified (January 2018)
[edit]Hello fellow Wikipedians,
I have just modified one external link on Sulfur dioxide. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20120723065412/http://www.cdph.ca.gov/pubsforms/Guidelines/Documents/fdb%20Sulfites.pdf to http://www.cdph.ca.gov/pubsforms/Guidelines/Documents/fdb%20Sulfites.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 09:56, 22 January 2018 (UTC)
Confusing/contradictory information about the structure
[edit]At the top of the sulfur dioxide properties table, there are images of the SO2 molecule shown as O=S=O, with two double bonds. But suddenly, in the "Structure and bonding" section, as it says - by the terms of a "valence bond theory approach considering just s and p orbitals" - there are images of the SO2 molecule shown as −O−S+=O ←→ O=S+−O−, suggesting that this is an average structure of SO2, and it says that the sulfur–oxygen bond has a bond order of 1.5. That contradicts to the image of the SO2 molecule shown as O=S=O, where the bond order is obviously 2. Now someone could yell that O=S=O and −O−S+=O are one of the many possible structures of SO2 molecule, but why to confuse readers by showing structures like O=S=O, without explaining from what theory the O=S=O structure is derived, and is the O=S=O structure an average in that other theory, or is it just one of the many possible non-average structures in the "valence bond theory approach considering just s and p orbitals"? Bernardirfan (talk) 12:29, 6 July 2020 (UTC)
- Ah the joy of being a critic of a complicated situation! --Smokefoot (talk) 17:16, 6 July 2020 (UTC)

Bernardirfan (talk) 22:29, 6 July 2020 (UTC)
Problematic statement in introduction
[edit]This article gets 1000+ views per day, so it is a high priority that the lead be written properly. Specifically, the recently added statement "Sulfur dioxide has pungent smell like nitric acid" needs a citation and grammatical improvement. I'm asking someone else to do it because I already removed the statement too many times (momentarily forgot about the 3-revert rule .. my apologies). MaxwellMolecule (talk) 00:12, 31 January 2021 (UTC)
- Wikipedia articles that use IUPAC spelling
- Wikipedia articles that use American English
- B-Class level-5 vital articles
- Wikipedia level-5 vital articles in Physical sciences
- B-Class vital articles in Physical sciences
- B-Class chemicals articles
- Mid-importance chemicals articles
- B-Class WPChem worklist articles
- B-Class WikiProject Volcanoes articles
- Mid-importance WikiProject Volcanoes articles
- All WikiProject Volcanoes pages
- B-Class Food and drink articles
- Low-importance Food and drink articles
- WikiProject Food and drink articles






