Talk:Chloralkali process
| This article is rated C-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||||||||||||
| ||||||||||||||||||
Chlor-Alkali Membrane Cell Process
[edit]Caustic soad (NaOH) and chlorine are produced by the electrolysis of an aqueous solution of sodium chloride (brine). Previous technologies used in the chlor-alkali process included the use of mercury and diaphragm cells. Due to pollution concerns, the mercury cell technology has nearly been displaced as there are less than 10 of these plants operating in the U.S. Diaphragm cell technology is still in use, but newer plants are turning to a newer technology because the older diaphragm cells utilitze asbestos in the cells. The increased economics of the new membrane process are also a deciding factor.
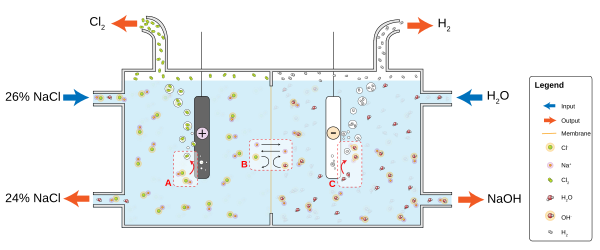
The newest chlor-alkali processes rely on an ion-exchange membrane to separate the sodium and chloride ions of the sodium chloride. The ion-exchange membranes typically fluoropolymer based and can contain sulfonic acid groups. Figure 1 represents a condensed overview of a membrane cell process.
From: http://www.cheresources.com/chloralk.shtml —Preceding unsigned comment added by Komada yako (talk • contribs)
Anodes
[edit]I don't think that it is correct to say that titanium can be used as an anode. Titanium quickly anodizes to the point that all current flow stops when used as an anode. It is commonly used as the cathode however in electrolytic processes. I have heard of nickel being used as an anode, but not in this situation... I don't think it would fair well in a chloride solution, which is extremely corrosive. It is my understanding that MMO (mixed metal oxide) anodes are used. These are inert conductive oxides placed on top of the titanium. --174.30.58.1 (talk) 09:25, 24 February 2011 (UTC)
- When I was young, I used to make little electrolysis cells, mostly to make hydrogen. I tried to make balloons, but I don't think I ever got it to go in. I used carbon anodes, using the carbon electrode from a carbon-zinc cell. That was for fun, not for industrial use, though. Gah4 (talk) 05:08, 14 March 2019 (UTC)
- Only 8 years late-Apparently the titanium is usually coated in RuO2/SnO2/IrO2. I just need to find a good reference for this Pelirojopajaro (talk) 21:09, 18 March 2019 (UTC)
chlorine demand
[edit]I thought that chlorine was in more demand than hydrogen or NaOH for use treating potable water in muni water supplies. —Preceding unsigned comment added by 50.80.150.100 (talk) 19:28, 12 April 2011 (UTC)
File:Chloralk2.gif Nominated for Deletion
[edit]
|
An image used in this article, File:Chloralk2.gif, has been nominated for deletion at Wikimedia Commons in the following category: Deletion requests December 2011
Don't panic; a discussion will now take place over on Commons about whether to remove the file. This gives you an opportunity to contest the deletion, although please review Commons guidelines before doing so.
This notification is provided by a Bot --CommonsNotificationBot (talk) 11:02, 31 December 2011 (UTC) |
need to cover industry, not just process
[edit]We have no article on the industry. This should be an article that explains the process a lot, but also discusses the size of the industry, geography, etc.TCO (Reviews needed) 21:28, 1 January 2012 (UTC)
File:Chloralkali membrane.svg to appear as POTD
[edit]Hello! This is a note to let the editors of this article know that File:Chloralkali membrane.svg will be appearing as picture of the day on May 2, 2013. You can view and edit the POTD blurb at Template:POTD/2013-05-02. If this article needs any attention or maintenance, it would be preferable if that could be done before its appearance on the Main Page. Thanks! — Crisco 1492 (talk) 22:48, 15 April 2013 (UTC)
Different layout of membrane process
[edit]While looking for details of the membrane, I found this: http://www.cheresources.com/chloralk.shtml which shows a significantly different layout of the membrane cell. ArthurDent006.5 (talk) 08:28, 7 January 2015 (UTC)
External links modified
[edit]Hello fellow Wikipedians,
I have just modified one external link on Chloralkali process. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20090518141937/http://electrochem.cwru.edu:80/encycl/art-b01-brine.htm to http://electrochem.cwru.edu/encycl/art-b01-brine.htm
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 21:04, 22 November 2016 (UTC)