Talk:Alkane/Archive 1
| This is an archive of past discussions about Alkane. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 |
Physical properties of alkanes as fuels
I think there should be a mention to the way the different alkanes are related to common fuels, and their physical properties. Methane is a gas at normal condition, and very difficult to liquify, (methane + ethane = natural gas? not sure), butane and propane are also gases, but they can be liquified rather easily, and so they are sold in cilinders, in liquid form. Those cilinders are easily recharged. pentane and hexane, i'm not sure, but heptane and octane are liquid in normal conditions, are main constituents of gasoline. Somewhere in the tens of carbons, the alkanes are solid in normal conditions (paraffine)....etc..
Substitution vs isomerization
Does methylpropane count as propane? The way the article's worded suggests that it is simply an isomer of propane, which is definitely isn't! -- Steinsky
standardization of chemical articles
I think there should be a standardized form of all chemical articles. It should include a chart with physical properties somewhere, and the same article sections. It would be a tough job to do, considering the vast number of chemicals in the database.
--Edsanville 20:56, 2 Jun 2004 (UTC)
- There is one, at WikiProject Chemistry, however, a template for organic compounds has yet to be established. Gentgeen 21:04, 2 Jun 2004 (UTC)
Smallest alkane that does not exist
What is the smallest integer Z where CZH2Z+2 does not exist?? 66.245.80.45 16:48, 18 Sep 2004 (UTC)
- He he this looks like a homework question. Okay, you still need to figure it out yourself, so I'm just helping with the arithmetics:
z CzH2z+2 Structure Does it exist? -------------------------------------------------------------- ... 3 C3H8 H3C-CH2-CH3 2 C2H6 H3C-CH3 1 CH4 CH4 0 H2 H2 -1 C-1 ? ...
- --Unconcerned 04:23, 24 Sep 2004 (UTC)
H2 is not considered an alkane because it has no carbon!
Suggest 5 possible wiki links and 2 possible backlinks for Alkane.
An automated Wikipedia link suggester has some possible wiki link suggestions for the Alkane article:
- Can link Melting point: ... * The density of an alkane is less than water density. * Melting point and boiling point increase with molecular weight and with l... (link to section)
- Can link boiling point: ...an alkane is less than water density. * Melting point and boiling point increase with molecular weight and with length of the main ... (link to section)
- Can link molecular weight: ... density. * Melting point and boiling point increase with molecular weight and with length of the main carbon chain.... (link to section)
- Can link carbon chain: ... increase with molecular weight and with length of the main carbon chain.... (link to section)
- Can link hydrogen atom: ...lysed with [[UV]]. '''2.''' Initiation step (slow step): a hydrogen atom is pulled off from methane... (link to section)
Additionally, there are some other articles which may be able to linked to this one (also known as "backlinks"):
- In Chloroform, can backlink aliphatic hydrocarbon: ...ause vomiting. [[Trichloroethylene]], a related halogenated aliphatic hydrocarbon, later supplanted chloroform as a safer alternative, though...
- In Water contact, can backlink saturated hydrocarbon: ...he pores, which creates a transition zone between the fully saturated hydrocarbon levels and the fully saturated water levels.
Notes: The article text has not been changed in any way; Some of these suggestions may be wrong, some may be right.
Feedback: I like it, I hate it, Please don't link to — LinkBot 11:29, 1 Dec 2004 (UTC)
Alkanes vs fats & oils
Alkanes differ only slightly from fats and oils. Some discussion of this, would be useful. —The preceding unsigned comment was added by 68.169.169.65 (talk)
Cracking and Reforming
This section doesn't mention reforming at all, only cracking. --82.43.150.220 19:38, 15 May 2006 (UTC)
Good article nomination failed
- WP:LEAD too short.
- The names of all alkanes end with -ane. ... why? Talk about the history or where the nomenclature comes from.
- Trivial names ... again why are these name kept or why do these compound have multiple names.
- Images imbedded in text isn't recommended, plus they need to have captions.
- Too many lists, at least in the beginning.
- References missing. Use the Cite.php method if possible because it is recommended.
- The line, Alkanes occur both on Earth and in the solar system, however only the first hundred or so, and even then mostly only in traces., doesn't make any sense ... first hundred of what, alkanes, branched alkanes or unbranched alkanes.
- methane and ethane are every day gases, at least methane is emitted by animals through their feces. That is much more relevant than knowing it is present on such comet that is not known to the general public.
- On Titan, the satellite of Saturn, it is believed that there were once large oceans of these and longer chain alkanes: smaller seas of liquid ethane are thought still to exist there. - This is trivia or it pertains tu such articles as Titan (moon) or ethane.
- ... produced primarily by forms of Archaea. - maybe should say ... produced primarily by organisms such as Archaea.
- Although they cannot be commercially exploited at the present time, the calorific value of the known methane hydrate fields exceeds the energy content of all the natural gas and oil deposits put together — methane extracted from methane hydrate is considered therefore a candidate for future fuels. - This should not be in the occurence section but in the Properties section.
- Occurence section and Alkanes in nature should be merged or be one under the other as they go toward a same goal, which is showing where these alkanes are present.
- In the Occurence section, the Today, the most important commercial sources ... text and what comes after should go into a section that explains why do we have the alkanes on the earth like Earth's alkane and its sources or so.
- ... within the individual fractions the boiling points lie closely together. - Some explanation on this point .would be greatly appreciated
- ... although the following demarcation is idealized and not perfect. - This statement is pov unless it is referenced or the word idealized is changed.
- The Preparation section is a bit short, needs images of the reactions or needs to be merged with the Purification section.
- The reactions should be labeled with names or be traced back to the publications where they were taken from.
Lincher 21:31, 4 June 2006 (UTC)
- trim down on conformation as it is already covered in Alkane stereochemistry, same goes for reactions of alkanes. Also too many subheaders V8rik 21:57, 4 June 2006 (UTC)
Nomenclature
It's already mentioned in the article that the first four members of the series don't follow the numerical prefix pattern. Would it be worth mentioning what the prefixes mean?
Briefly: Meth- comes from Greek methu, meaning wine; eth- is linked to ether; prop- comes from pro and pion, Greek words meaning "forward" and "fat", respectively (propane is related to fatty acids); and but- comes from Latin butyrum, meaning butter.
Found this information on the Online Etymology Dictionary.
Maerk 03:12, 21 January 2007 (UTC)
Nomenclature (continued)
Wikipedia should name all hydrocarbons the IUPAC systematic way. What do i want to... 10:27 25 November '09 —Preceding undated comment added 10:28, 25 November 2009 (UTC).
Basis for a new intro?
Alkanes are chemical compounds that consists only of the elements carbon (C) and hydrogen (H) (i.e. hydrocarbons), wherein each of these atoms are linked together exclusively by single bonds (i.e. saturated compounds).
Since these chemical compounds are saturated, each carbon atom must have 4 bonds (either C-H or C-C bonds), and each hydrogen atom must be joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. Typically the number of carbon atoms is often used to define the size of the alkane (e.g. C2-alkane).
Alkanes can be linear (general formula CnH2n+2, where n>0) where the carbon atoms are joined in a snake like structure, cyclic (general formula CnH2n where n>2) where the carbon backbone is linked so as to form a loop, or branched (general formula CnH2n, where n>3) where the carbon backbone splits off in one ore more directions.
The simplest possible alkane (the parent molecule) is methane, CH4. There is no limit to the number of carbon atoms that can be linked together, the only limitation being that the molecule is saturated and is a hydrocarbon. Saturated oils and waxes are example of larger alkanes where the number of carbons in the carbon backbone tend to be great than 10.
The trivial name (non-systematic) for alkanes is paraffins, or collectively as the paraffin series. Trivial names for compounds usually are a historical artefact, often steming from industrial practices. The term paraffins almost certainly stems from the petrochemical industry. Branched-chain alkanes are called isoparaffins. Cycloalkanes (also called naphthenes) are alkanes that containing one or more rings. The use of the term "paraffin" is a general term and often does not distinguish between a pure compounds and mixtures of isomers with the same chemical formula (i.e. like a chemical anagram) e.g. pentane and isopentane. --Quantockgoblin 22:55, 5 February 2007 (UTC)
- Sound's good. I've added it in. --Rifleman 82 23:37, 5 February 2007 (UTC)
Nomenclature of alkanes
The Geneva system is based on identifying hydrocarbon chains, most of which are named systematically with a Greek numerical prefix denoting the number of carbons and the suffix "-ane".[citation needed]
Wilhelm Hofmann suggested systematizing nomenclature by using the whole sequence of vowels a, e, i, o and u to create suffixes -ane, -ene, -ine (or -yne), -one, -une, for the hydrocarbons. Only the first three caught on for naming hydrocarbons with single, double and triple bonds.
Linear Alkanes
Straight-chain alkanes are sometimes indicated by the prefix n- (for normal) where a non-linear isomer exists. Although this is not strictly necessary, the usage is still common in cases where there is an important difference in properties between the straight-chain and branched-chain isomers: e.g. n-hexane or cyclohexane 2- or 3-methylpentane.
The first four members of the series (in terms of number of carbon atoms) are named as follows:
Alkanes with five or more carbon atoms are named by adding the suffix -ane to the appropriate numerical multiplier with elision of a terminal -a- from the basic numerical term. Hence, pentane, C5H12; hexane, C6H14; heptane, C7H16; octane, C8H18; etc. For a more complete list, see List of alkanes.
Branched alkanes
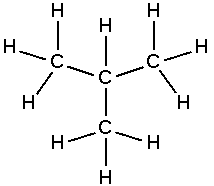
Simple branched alkanes often have a prefix "iso-" to distinguish them from linear alkanes.
The key steps in the naming of more complicate branched alkanes are as follows:
- Identify the longest linear chain of carbon atoms.
- This portion is named as if it where a liner alkane (i.e. 3 linear carbon atoms would be a propane).
- One of the terminal ends of this chain is numbered 1. The terminus which has the fewest carbon atoms from the branch is numbered 1.
- the nomenclature is determined for the branching group in a similar fashion as above.
- the compound is then named (branching number e.g. 2)-(name of branching group, e.g. methyl)-(name of longest linear chain, e.g. propane) so as to give you the alkane's name, e.g. 2-methyl-propane which is shown right.
Cyclic alkanes
Simple cyclic alkanes often have a prefix "cyclo-" to distinguish them from linear or branched alkanes. Cycloalkanes are named as per linear alkanes with respect to the number of carbon atoms e.g. cyclopentane is a alkane with 5 carbon atoms joined up in a five membered ring.
Trivial names
The trivial (non-systematic) name for alkanes is "paraffins". Collectively, alkanes are known as the paraffin series. Trivial names for compounds are usually historical artefacts. They were coined before the development of systematic names, and have been retained due to familiar usage in industry.
The term paraffins almost certainly stems from the petrochemical industry. Branched-chain alkanes are called isoparaffins. Cycloalkanes (also called naphthenes) are alkanes that containing one or more rings. The use of the term "paraffin" is a general term and often does not distinguish between a pure compounds and mixtures of isomers with the same chemical formula (i.e. like a chemical anagram) e.g. pentane and isopentane.
- Examples
The following trivial names are retained in the IUPAC system:
- isobutane for 2-methylpropane
- isopentane for 2-methylbutane
- neopentane for 2,2-dimethylpropane
- Will wait for at least one postive review before replacing text -- Quantockgoblin 00:15, 6 February 2007 (UTC)
alkane redirects
I noticed that the following alkanes mention in this article:
Just have a rediredt back to alkane, which is not very helpful - delete redirect pages? — The preceding unsigned comment was added by Quantockgoblin (talk • contribs) 15:00, 6 February 2007 (UTC).
Isomers list
In the "Isomerism" section, the text says "for alkanes with 1 to 12 carbon atoms, the number of isomers equals" but the subsequent list only contains C1-C4 and C12. Should we include C5-C11 as the intro says the list will contain? Or should we add a C15ish entry and adjust the text to indicate simply "as the number of carbons increases, the number of possible isomers increases rapidly" (or something...not sure two "increases" is good there)? I sorta like the latter, as it illustrates the point about structural possibilities and an exhaustive list doesn't really serve a purpose. DMacks 03:08, 6 April 2007 (UTC)
Mathematicians have this vertical ...
it looks like this . .
. .
. .
when denoting series, while leaving out some entries. How about that? --Rifleman 82 07:13, 6 April 2007 (UTC)
Looks good as-is, but the vertical-elipsis thingy would be fine too. If you like <math> I guess:) Or a wikitable with the ⋮ character. DMacks 08:10, 6 April 2007 (UTC)
- Okay, that special character makes a mess of the Firefox text-editor box, so forget that one. Could use inline LaTeX to get it:
- C1: 1 (methane)
- C2: 1 (ethane)
- C3: 1 (propane)
- C4: 2 (n-butane, isobutane)
- C12: 355
- C32: 27,711,253,769
- C60: 22,158,734,535,770,411,074,184
- Bullets look strange there. LaTeX matrix and wiki table both give fairly large layout, over-emphasizing this relatively minor (IMO) issue. DMacks 20:14, 6 April 2007 (UTC)
- Would this be any good?
| CH4 | Methane | C4H10 | n-Butane | C7H16 | n-Heptane | C10H22 | n-Decane |
| C2H6 | Ethane | C5H12 | n-Pentane | C8H18 | n-Octane | C11H24 | n-Undecane |
| C3H8 | Propane | C6H14 | n-Hexane | C9H20 | n-Nonane | C12H26 | n-Dodecane |
These isomers are a specific type of isomer, the constitutional isomer. There are also stereo isomers possible in chiral hydrocarbons. I think that this should be updated. --Satanorsanta 19:43, 19 April 2007 (UTC)
I have added a note to the entry for C60 22,158,734,535,770,411,074,184 isomers to indicate many of which are not stable. The reason for this is simple: The mathematical formula used to generate that number neglects the fact that arbitrary branching that many times is not actually feasible due to geometric constraints; the carbon side chains will get in the way of each other. In many of those cases I doubt that there is even a way to imagine forming the molecule, let alone have it be stable, but thats another debate. —Preceding unsigned comment added by Zaphraud (talk • contribs) 14:53, 24 June 2008 (UTC)
page loads with gibberish
Hi!
I'm writing here because I don't know were else to write, but somehow when i went to this page to read about alkanes, the first paragraph was a string of gibberish. Thought I'd flag it.
--mk
- Thanks mk, crisis averted, page restored. V8rik 17:00, 8 June 2007 (UTC)
Alkanes can be cyclic?
The current version explicitly states that alkanes CAN be cyclic. In English language, it seems only logical to say cyclic alkanes are alkanes, but I'd like to point out that, according to the IUPAC, alkanes are Acyclic branched or unbranched hydrocarbons [1], and so, technically, it CANNOT be cyclic at all. So it seems to me at least... — Gyopi 20:18, 20 June 2007 (UTC)
- So, I've replaced the term "alkanes" with "hydrocarbons" in the introduction part and the "cyclic alkanes" section. Also changed was "cyclic alkanes" section itself, to "cycloalkanes". I thought it would be more accurate from the point of the view of the IUPAC nomenclature. However, Gyopi pointed out that (in the talk page of the ja alkane), though the term "cyclic alkane" is incorrect in the IUPAC, it still being used generally as the word expressing hydrocarbons having cyclic structure. Gyopi also suggested that "cyclic alkanes" should be left anywhere in the article, because readers may expect and/or do serch/reserch with the term. Then, I think write it back in the section is okay. How about the section title? One more point, if we set the redirect cyclic alkane, where it should be refer (alkane#cyclic alkane/cycloalkane or just cycloalkane)? --Calvero JP 14:01, 23 June 2007 (UTC)
- Hi. Thanks, Calvero... for your commitment.
- Well, while I fully acknowledge the term "cyclic alkanes" technically doesn't make any sense (it would only mean "cyclic acyclic alkanes"), I must point out that just because it is weird IUPAC-wise doesn't mean we MUST NOT use it. The definition by IUPAC should be respected, but we can (and maybe should) note that cycloalkanes are often (incorrectly, if you put that way) called cyclic alkanes, which can't be helped because they are just called that way in English, no matter what IUPAC says. As you can see, the terms cyclic OR acyclic alkanes are still used in the current version (you didn't replace them for some reason). And if we accept that reality, i.e. the technically weird expression "(a)cyclic alkanes", then it seems almost inevitable that one says "alkanes CAN be cyclic" just like "a cyclic group is a group" in math. That's the dilemma, and that's why I noted this question here, before actually editing anything.
- Personally, I wouldn't mind banning the term "cyclic alkanes". However, it is possible that someone would like to know about "normal" alkanes and cyclic alkanes, and it would be better if Wikipedia could answer at least a little (like, "See cycloalkanes") than it couldn't answer at all. Maybe we could use some flexibility, not just mechanically replacing every occurrence of "cyclic alkanes"...
- Any further input would be appreciated. — Gyopi 03:35, 24 June 2007 (UTC)
- The results from Goolge search show us "cyclic alkane" is quite general, and even "aromatic alkane" is used in published papers. The term "alkane" quite often used as the same meaning of "hydrocarbon". I realized that the replacement I've made was somewhat ridiclous — however, I believe my edit diidn't led the article to wrong direction, so I won't revert my edit at this time. Anyway, any other opinion/modification will be welcomed. --Calvero JP 07:14, 24 June 2007 (UTC)
- Okay, if you say so... But anyway, editing is needed to fix grammar. if no one else is going to do that, I'll have to do that myself, but I really appreciate it if anyone could help us. Anyone more suitable than me. — Gyopi 00:47, 26 June 2007 (UTC)
- Go ahead please, and I will not provide any objection to further edits about this subject. In addition to the grammer problems (sorry), I feel that I can't improve this article anymore. If I get some idea I'll be back. --Calvero JP 16:57, 27 June 2007 (UTC)
- Okay, if you say so... But anyway, editing is needed to fix grammar. if no one else is going to do that, I'll have to do that myself, but I really appreciate it if anyone could help us. Anyone more suitable than me. — Gyopi 00:47, 26 June 2007 (UTC)
- The results from Goolge search show us "cyclic alkane" is quite general, and even "aromatic alkane" is used in published papers. The term "alkane" quite often used as the same meaning of "hydrocarbon". I realized that the replacement I've made was somewhat ridiclous — however, I believe my edit diidn't led the article to wrong direction, so I won't revert my edit at this time. Anyway, any other opinion/modification will be welcomed. --Calvero JP 07:14, 24 June 2007 (UTC)
I've made an executive decision that cyclic alkane should redirect to cycloalkane; if anyone doesn't like that, don't forget that this is a wiki, {{sofixit}} ;) I'll take a look at the grammar of this article this evening off wiki to try to give Gyopi a hand. Physchim62 (talk) 17:36, 27 June 2007 (UTC)
- Thanks Psychim, I did something real quick, counting on your help :) Calvero, you should give yourself some credit. Your edit is essentially a good one. You don't have to be sorry at all. It's just grammar (like, hydrocarbons is->hydrocarbons are) and my English sux too. — Gyopi 21:49, 27 June 2007 (UTC)
- The article was originally written totally assuming that alkane can be cyclic. Several (sub)sections are affected by that, directly or indirectly, deeply or subtly, and just updating the definition doesn't work. It's not easy for me to change the definition drastically while (1) maintaining the integrity and (2) considering both the IUPAC definition AND non-technical but quite common usage ("cyclic alkanes"). Honestly, I'm not the right person to edit this GA candidate thing. Please, someone help... — Gyopi 08:52, 29 June 2007 (UTC)
- I'm now back from oving house, so I'll see what I can do: I agree that it's a big job though. Incidentally, I didn't find much in the way of really bad grammar when I checked last week—maybe someone got there first! Physchim62 (talk) 17:34, 1 July 2007 (UTC)
- Yes, Rifleman 82, a bot, and I edited this thing after Calvero JP (I'm not saying "it's not easy for me" even without trying). I took the liberty to do what seemed best for me, quickly but boldly, partly inspired by your first comment. My points are basically (1) and (2) above. Calvero practically 'banned' the term "cyclic alkane" and like I said already, I'd disagree with that. But I may be wrong. — Gyopi 09:30, 2 July 2007 (UTC)
- I'm now back from oving house, so I'll see what I can do: I agree that it's a big job though. Incidentally, I didn't find much in the way of really bad grammar when I checked last week—maybe someone got there first! Physchim62 (talk) 17:34, 1 July 2007 (UTC)
I just did a few more tweaks and I hope now everything is decent about this, more or less. Note that I also replaced 'aromatic alkanes' with 'aromatic hydrocarbons' (one occurrence). Anyone who can help, please, double-check spelling, grammar, style, etc. and do feel free to re-edit as much as needed to make the rewritten parts flow better. — Gyopi 18:47, 4 July 2007 (UTC) PS. This is the diff. [2] Shorter than I thought. — Gyopi 18:50, 4 July 2007 (UTC)
Physical properties of Alkanes
Hi all, i tried to create a table of physical properties of some alkanes. The problem i am facing is that anywhere i may place (paste) the table on the article, after i see the preview, it shows it at the bottom of the article. If there are no objections to the table can someone please help placing it to a more correct position ? (under physical properties would be ideal in my opinion). Thanks Meander₪ 10:41, 10 May 2008 (UTC)
- fixed thanks Meander₪ 18:27, 10 May 2008 (UTC)
preparation of alkanes
Alkanes can be prepared by following methods. (1)Hydrogination of Alkenes.
hydrogination means addition of Hydrogen to multiple bond is called Hydrogenation. these hydrogen break the multiple bond and form single bond. like
C2H4 and H2 reac with each other to form C2H6. —Preceding unsigned comment added by 119.154.51.114 (talk) 01:57, 2 June 2009 (UTC)
alkanes are the compound that are saturated aur have a sigma bond which indicates dat it has a stable compound and cant react with other compound......this shows its stability with other..meanz it has a single sigma bond ...so its stable........
MELTING POINT
the melting point of alkanes show varaiationsssss —Preceding unsigned comment added by 58.27.152.14 (talk) 15:52, 15 August 2009 (UTC)
3rd paragraph of introduction
Are other editors satisfied with the introduction, especially the 3rd paragraph as it currently reads? Shanata (talk) 06:53, 15 October 2009 (UTC)
HOW ARE ALKANES USEFULL AND WHERE WOULD THEY BE FOUND ???????? —Preceding unsigned comment added by 94.192.35.24 (talk) 13:23, 7 April 2010 (UTC)
Accuracy of monobromination image
In this edit, an editor pointed out that the statistical distributions of the alkane mix in the image [3] are incorrect. I think he/she might be right, should the image be changed or is the note that the editor left enough? 69.91.130.96 (talk) 22:28, 5 October 2010 (UTC)
- There are 8 hydrogen atoms in propane. If each hydrogen atom is equally likely to be replaced by bromine (a statistical distribution), then the product distribution should be 2/8 isopropyl bromide, and 6/8 n-propyl bromide, i.e. a 25%-75% distribution, as you mention.
- Am I right?
'Occurrence' image
Is there a reason why an image of Jupiter is featured, as opposed to one of Uranus? Uranus is a better representative, as it has the highest concentration of alkanes as listed at the top of the section. Plasmic Physics (talk) 08:31, 5 April 2013 (UTC)
Untitled
+++ cycloalkanes +++ I'll accept the writers word for it that cycloalkanes are not a sub-category of alkanes **according to IUPAC**. There is abundant *technical* literature that disagrees (and treats them as a sub-category). (some of it, I'll admit, is older). So, if the consensus is that cycloalkanes are not alkanes then perhaps less should be said of them in this article. Cycloalkanes certainly do NOT have the formula C(n)H(2n). For instance the tetrahedral structure C4H4 or add a CH2 group onto each vertice, C10H16. The formula given is only correct for mono-cyclic alkanes. Carbon nanotubes (saturated with some pendant H's) also are "cycloalkanes" - or perhaps rationalization of nomenclature (a la IUPAC) has not had a chance to catch up... Anyway, if you want to exclude cyclics, rings, tubes, balls, etc. you should do that in the LEAD and at most put a small section explaining why and what.69.40.241.198 (talk) 03:50, 23 February 2011 (UTC)
- Let's go along with noncyclicity so the statement that the molecular mass adds 14 with each carbon will be true from the ground up (starting with methane). Obviously it's not true if arbitrary cyclicity is allowed. (It is true if we allow any base hydrocarbon, single-bonded or not, and add methyl groups without adding cycles; any such series will satisfy the +14 rule. The compounds needn't be alkanes in any definition.) Meanwhile, I removed the cyclicity conflict statement in the introduction, and let's await further input from experts. Zaslav (talk) 18:52, 21 August 2011 (UTC)
"Paraffin" in the UK
Perhaps I wasn't careful enough in my reading, but it seems that British usage of the word "paraffin" for what is commonly known in the USA as "kerosene" could have been mentioned.
Best regards, user Nikevich (DHCP; sorry.) 72.74.254.33 (talk) 21:51, 8 September 2012 (UTC)
It's confusing to initially say: "Acyclic alkanes"
In the first paragraph of the Intro are the sentences: "...Alkanes consist only of hydrogen and carbon atoms and all bonds are single bonds. [1] Acyclic alkanes have the general chemical formula CnH2n+2...." Then, in the middle of the fourth paragraph it says: "... the only limitation being that the molecule is acyclic...." Finally, under Nomenclature, Cyclic alkanes, it says: "So-called cyclic alkanes are, in the technical sense, not alkanes, but cycloalkanes...."
In common (but technically incorrect) use, therefore, "alkane" might informally include cyclic compounds. Nevertheless, I would like to see those two sentences of the Intro read something like: "...Alkanes consist only of hydrogen and carbon atoms and all bonds are single bonds. [1] Alkanes (technically, always acyclic) have the general chemical formula CnH2n+2...."
The way that the second of these two sentences currently reads, it gives the reader (who may not read farther than that first paragraph) the impression that the term "alkane" formally (not merely in some informal use) does include compounds that are not acyclic, when, in fact, the article really wants to help the reader understand and use the term to include only acyclic compounds. Wikifan2744 (talk) 00:44, 8 August 2014 (UTC)
- Yes. Suggest you copy edit it.Plantsurfer (talk) 09:55, 8 August 2014 (UTC)
Done. Glad you agree. Thanks. Wikifan2744 (talk) 01:39, 29 August 2014 (UTC)
Viscosity
Dont we need to put in the viscosity of the liquid alkanes? AS these are sometimes used as lubricants, I think that would be a good idea.--213.205.192.13 (talk) 01:20, 31 January 2016 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified one external link on Alkane. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive http://web.archive.org/web/20131029192647/http://nsdl.niscair.res.in/bitstream/123456789/777/1/Revised+organic+chemistry.pdf to http://nsdl.niscair.res.in/bitstream/123456789/777/1/Revised+organic+chemistry.pdf
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 10:38, 21 July 2016 (UTC)
Why Methane, Ethane, Propane, and Butane instead of Unane, Diane, Triane, and Tetrane?
Why are the alkanes 1-4 called Methane, Ethane, Propane, and Butane instead of Unane, Diane, Triane, and Tetrane? Can someone explain this? Thanks. 24.150.217.182 (talk) 13:33, 17 June 2017 (UTC)
- The prefixes meth-, eth-, prop-, and but- were all in common use before chemical nomenclature was formally systematized. When IUPAC developed their nomenclature, they decided to retain some of the most commonly used terms, even when they were not consistent with the system being developed, just because they were in use so widely. A couple of seconds on Google leads to these pages which describe how each of those four terms originated: Methyl_group#Etymology, Alkyl#Etymology, wikt:propionic_acid#Etymology, Butyl_group#Etymology. -- Ed (Edgar181) 16:05, 17 June 2017 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified 2 external links on Alkane. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20120202091842/http://www.chem.yale.edu/~chem125/125/history99/5Valence/Nomenclature/alkanenames.html to http://www.chem.yale.edu/~chem125/125/history99/5Valence/Nomenclature/alkanenames.html
- Added archive http://www.webcitation.org/6F5FQG2OP?url=http://www.geo.uni-frankfurt.de/iau/epos/Gruppenintern/Kesselmeier___Staudt_JAC_1999.pdf to http://www.geo.uni-frankfurt.de/iau/epos/Gruppenintern/Kesselmeier___Staudt_JAC_1999.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 05:07, 26 July 2017 (UTC)
acyclic?
Is it necessary to complicate the definition with such precision? Isn't it a given? Asking generally, no chemistry professor is I.. --188.120.138.62 (talk) 13:09, 7 August 2017 (UTC)
Add source to a part in "Chemical properties"->"Other reactions"
In the section "Other reactions" in "Chemical properties", it's written that 'the fermentation of alkanes to carboxylic acids is of some technical importance', however no source is given for the same. Of late I've been trying to find something to find something on oxidation of alkanes, so it would be helpful of someone sourced that particular line. Mr.Mog (talk) 06:09, 19 March 2019 (UTC)
What's the ethymological root alk.. of this word?
150.227.15.253 (talk) 17:04, 18 March 2021 (UTC)
Is the term "paraffin" really that important/frequent?
The very first sentence of the introductory paragraph does not seem to me to benefit from including the alternative name.
Addition of explaining that alternative name seems downright excessive.
I suggest that the article might be improved by moving "paraffin (a historical trivial name that also has other meanings)" to somewhere else.
2601:1C1:C180:4F40:0:0:0:452D (talk) 01:56, 13 February 2022 (UTC) A Nony Mouse
Chemistry
How can we draw essay structure of the compounds and molecular formula 2402:3A80:1CBD:BAB:0:51:833:A501 (talk) 13:41, 11 December 2022 (UTC)
"Test for alkanes" listed at Redirects for discussion
![]() An editor has identified a potential problem with the redirect Test for alkanes and has thus listed it for discussion. This discussion will occur at Wikipedia:Redirects for discussion/Log/2023 January 8 § Test for alkanes until a consensus is reached, and readers of this page are welcome to contribute to the discussion. Mdewman6 (talk) 01:21, 8 January 2023 (UTC)
An editor has identified a potential problem with the redirect Test for alkanes and has thus listed it for discussion. This discussion will occur at Wikipedia:Redirects for discussion/Log/2023 January 8 § Test for alkanes until a consensus is reached, and readers of this page are welcome to contribute to the discussion. Mdewman6 (talk) 01:21, 8 January 2023 (UTC)
Chemistry
how many hydrogen atoms are there in ethane 41.210.146.173 (talk) 12:39, 13 June 2023 (UTC)
Good stuff?
I removed this discussion: Due to the subtlety of this effect, the exact reasons for this rule have been vigorously debated in the chemical literature and is yet unsettled. Several explanations, including stabilization of branched alkanes by electron correlation,[1] destabilization of linear alkanes by steric repulsion,[2] stabilization by neutral hyperconjugation,[3][4] and/or electrostatic effects[5] have been advanced as possibilities. The controversy is related to the question of whether the traditional explanation of hyperconjugation is the primary factor governing the stability of alkyl radicals.[6][2] --Smokefoot (talk) 17:43, 23 November 2023 (UTC)
Alkane
alkane information and examples 2409:40F0:F:4C5D:1890:D6FF:FEA6:4487 (talk) 13:31, 30 September 2024 (UTC)
- ^ Wodrich, Matthew D.; Wannere, Chaitanya S.; Mo, Yirong; Jarowski, Peter D.; Houk, Kendall N.; Schleyer, Paul von Ragué (2007). "The Concept of Protobranching and Its Many Paradigm Shifting Implications for Energy Evaluations". Chemistry – A European Journal. 13 (27): 7731–7744. doi:10.1002/chem.200700602. ISSN 1521-3765. PMID 17607688.
- ^ a b Gronert, Scott (1 February 2006). "An Alternative Interpretation of the C−H Bond Strengths of Alkanes". The Journal of Organic Chemistry. 71 (3): 1209–1219. doi:10.1021/jo052363t. ISSN 0022-3263. PMID 16438539.
- ^ Kemnitz, Carl R. (2013). "Electron Delocalization Explains much of the Branching and Protobranching Stability". Chemistry – A European Journal. 19 (33): 11093–11095. doi:10.1002/chem.201302549. ISSN 1521-3765. PMID 23868617.
- ^ Cite error: The named reference
Alabugin-2016was invoked but never defined (see the help page). - ^ Ess, Daniel H.; Liu, Shubin; De Proft, Frank (16 December 2010). "Density Functional Steric Analysis of Linear and Branched Alkanes". The Journal of Physical Chemistry A. 114 (49): 12952–12957. Bibcode:2010JPCA..11412952E. doi:10.1021/jp108577g. ISSN 1089-5639. PMID 21086970.
- ^ Ingold, K. U.; DiLabio, Gino A. (1 December 2006). "Bond Strengths: The Importance of Hyperconjugation". Organic Letters. 8 (26): 5923–5925. doi:10.1021/ol062293s. ISSN 1523-7060. PMID 17165895.

