Substituted phenethylamine
This article needs additional citations for verification. (August 2014) |
| Substituted phenethylamine | |
|---|---|
| Drug class | |
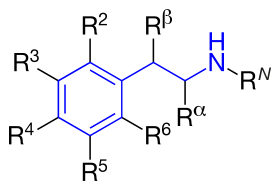 The structural formula of phenethylamine with marked substitution points. Phenethylamine is obtained when R2=R3=R4=R5=R6=RN=Rα=Rβ=H. | |
| Class identifiers | |
| Chemical class | Substituted derivatives of phenethylamine |
| Legal status | |
| In Wikidata | |
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure;[note 1] the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. Phenylethylamines are also generally found to be central nervous system stimulants with many also being entactogens/empathogens, and hallucinogens.
Structural classification
[edit]
The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH) group via a two-carbon sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms. Several classes of substances can be considered phenylethylamine derivatives such as Substituted amphetamines, where there is a methyl group substituted at the alpha position on the ethyl chain, Substituted methylenedioxyphenethylamines, where a methylenedioxy group is joined at the 3 and 4 positions on the phenyl ring, and Substituted cathinones, which have a carbonyl group substituted at the beta position on the ethyl chain, most of which also have a methyl group substituted at the alpha positioning making most cathinones substituted amphetamines as well.
Pharmacology
[edit]Most substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., 3,4,5-trimethoxyphenethylamine a.k.a. mescaline), 2,5-dimethoxy-4-methylamphetamine a.k.a. DOM), entactogen (e.g. MDA), appetite suppressants (e.g. phentermine), nasal decongestants and bronchodilators (e.g., levomethamphetamine and pseudoephedrine), antidepressants (e.g. bupropion and phenelzine), antiparkinson agents (e.g., selegiline), and vasopressors (e.g., ephedrine), among others.[1][2] Many of these psychoactive compounds exert their pharmacological effects primarily by modulating monoamine neurotransmitter systems; however, there is no known mechanism of action or biological target that is common to all members of this subclass.[medical citation needed]
Examples
[edit]Numerous endogenous compounds – including hormones, catecholamines such as dopamine and noradrenaline, and many trace amines (e.g. adrenaline, phenethylamine itself, tyramine, thyronamine, and iodothyronamine) – are substituted phenethylamines. Several notable recreational drugs, such as MDPV (Monkey Dust), MDMA (ecstasy), methamphetamine, and cathinone, are also members of the class. Many well-known prescription drugs are from the phenylethylamine class such as Adderall which uses Amphetamine, Desoxyn which uses methamphetamine, and Sudafed which uses pseudoephedrine.
List of substituted phenethylamines
[edit]| Chemical
Structure |
Short Name | RN | Rα | Rβ | R2 | R3 | R4 | R5 | Full Name | Biologic activity |
|---|---|---|---|---|---|---|---|---|---|---|
| meta-Tyramine | OH | 3-hydroxyphenethylamine | Trace amine | |||||||
| para-Tyramine | OH | 4-hydroxyphenethylamine | Trace amine | |||||||
| Dopamine | OH | OH | 3,4-dihydroxyphenethylamine | Catecholamine neurotransmitter | ||||||
|
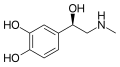
Epinephrine (Adrenaline) | CH3 | OH | OH | OH | β,3,4-trihydroxy-N-methylphenethylamine | Catecholamine neurotransmitter/Fight or Flight hormone | |||

|
Norepinephrine (Noradrenaline) | OH | OH | OH | β,3,4-trihydroxyphenethylamine | Catecholamine neurotransmitter/Fight or Flight hormone | ||||

|
Norfenefrine | OH | OH | β,3-dihydroxyphenethylamine | Trace amine | |||||
|
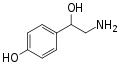
para-Octopamine | OH | OH | β,4-dihydroxyphenethylamine | Trace aminergic α-adrenoceptor agonist | |||||

|
Oxidopamine | OH | OH | OH | 2,4,5-trihydroxyphenethylamine | neurotoxic agent for the dopamine and norepinephrine receptors | ||||

|
Phenylephrine | CH3 | OH | OH | β,3-dihydroxy-N-methylphenethylamine | α-adrenergic agonist; decongestant | ||||
|
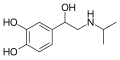
Isoprenaline | CH(CH3)2 | OH | OH | OH | β,3-dihydroxy-N-isopropylphenethylamine | β-adrenergic agonist; decongestant | |||
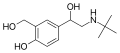
|
Salbutamol | C(CH3)3 | OH | CH2OH | OH | β,4-dihydroxy-3-hydroxymethyl-N-tert-butylphenethylamine | Short-action β2-adrenergic agonist | |||

|
β-Methylphenethylamine | CH3 | β-methylphenethylamine | Stimulant | ||||||

|
Amphetamine | CH3 | α-methylphenethylamine | Monoamine releasing agent; Stimulant | ||||||
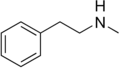
|
N-Methylphenethylamine | CH3 | N-methylphenethylamine | Trace amine; endogenous amphetamine isomer | ||||||

|
N,N-Dimethylphenethylamine | (CH3)2 | N,N-dimethylphenethylamine | Trivial effects (used as a food additive and flavoring agent) | ||||||
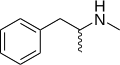
|
Methamphetamine | CH3 | CH3 | N-methylamphetamine; N,α-dimethylphenethylamine | Monoamine releasing agent; stimulant; neurotoxin | |||||
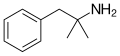
|
Phentermine | (CH3)2 | α-methylamphetamine; α,α-dimethylphenethylamine | Stimulant, anorectic | ||||||
|
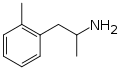
Ortetamine | CH3 | CH3 | 2-methylamphetamine; 2,α-dimethylphenethylamine | Stimulant, anorectic | |||||
|
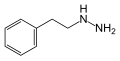
Phenelzine | NH2 | β-phenylethylhydrazine | Monoamine oxidase inhibitor | ||||||
|
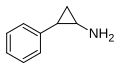
Tranylcypromine | -CH2- | 2-phenylcyclopropylamine | Monoamine oxidase inhibitor | ||||||

|
Selegiline | -CH2-C≡CH | CH3 | N,α-dimethyl-N-2-propynylphenethylamine | MAO-B selective monoamine oxidase inhibitor | |||||

|
Methylphenidate | -CH2-CH2-CH2-CH2- | C(OCH3)=O | N,α-butylene-β-methoxycarbonylphenethylamine | NDRI; Stimulant | |||||

|
Ephedrine / Pseudoephedrine | CH3 | CH3 | OH | N-methyl-β-hydroxyamphetamine | Releasing agent; stimulant; decongestant | ||||

|
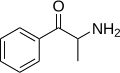
Cathine | CH3 | OH | d-β-hydroxyamphetamine | Moderately selective norepinephrine releasing agent | |||||
|
Cathinone | CH3 | =O | β-ketoamphetamine | Selective norepinephrine and dopamine releasing agent | |||||
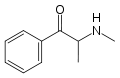
|
Methcathinone | CH3 | CH3 | =O | N-methylcathinone | Selective norepinephrine and dopamine releasing agent | ||||
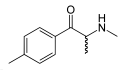
|
Mephedrone | CH3 | CH3 | =O | CH3 | 4-methylmethcathinone | Stimulant, unknown pharmacodynamic actions | |||
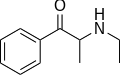
|
Ethcathinone | CH2CH3 | CH3 | =O | N-ethylcathinone | Stimulant and norepinephrine releasing agent | ||||
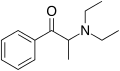
|
Amfepramone (diethylpropion) | C2H5, C2H5[note 2] | CH3 | =O | N-diethyl-β-ketoamphetamine | Anorectic | ||||
|
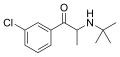
Bupropion | C(CH3)3 | CH3 | =O | Cl | 5-chloro-N-tert-butyl-β-ketoamphetamine | NDRI | |||
|
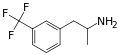
Norfenfluramine | CH3 | CF3 | 3-trifluoromethyl-amphetamine | SSRA | |||||

|
Fenfluramine | CH2CH3 | CH3 | CF3 | 3-trifluoromethyl-N-ethylamphetamine | SSRA | ||||
| 5-APB | CH3 | -CH=CH-O- | 5-(2-aminopropyl)benzofuran | Stimulant, entactogen | ||||||
| 6-APB | CH3 | -O-CH=CH- | 6-(2-aminopropyl)benzofuran | Stimulant, entactogen | ||||||
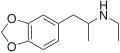
| MDA | CH3 | -O-CH2-O- | 3,4-methylenedioxy-amphetamine | Stimulant, psychedelic, entactogen | ||||||
|
MDEA | CH2CH3 | CH3 | -O-CH2-O- | 3,4-methylenedioxy-N-ethylamphetamine | Psychedelic, entactogen, and releasing agent | ||||

|
MDMA | CH3 | CH3 | -O-CH2-O- | 3,4-methylenedioxy-N-methylamphetamine | Psychedelic, entactogen, and releasing agent | ||||

|
MDMC | CH3 | CH3 | =O | -O-CH2-O- | 3,4-methylenedioxymethcathinone | Psychedelic, entactogen, and releasing agent | |||

|
MMDA | CH3 | -O-CH2-O- | OCH3 | 5-methoxy-3,4-methylenedioxy-amphetamine | Stimulant, psychedelic and entactogen | ||||

|
MMDMA | CH3 | CH3 | -O-CH2-O- | OCH3 | 5-methoxy-3,4-methylenedioxy-N-methylamphetamine | Psychedelic, entactogen, and releasing agent | |||

|
Lophophine | -O-CH2-O- | OCH3 | 5-methoxy-3,4-methylenedioxyphenethylamine | Psychedelic and entactogen | |||||
|
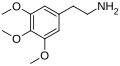
Mescaline | OCH3 | OCH3 | OCH3 | 3,4,5-trimethoxyphenethylamine | Psychedelic and entactogen | ||||

|
Proscaline | OCH3 | OCH2CH2CH3 | OCH3 | 2-(3,5-dimethoxy-4-propoxyphenyl)ethanamine | Psychedelic and entactogen | ||||

|
Metaescaline | OCH2CH3 | OCH3 | OCH3 | 2-(3-ethoxy-4,5-dimethoxyphenyl)ethanamine | Psychedelic and entactogen | ||||
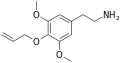
|
Allylescaline | OCH3 | OCH2CH1CH2 | OCH3 | 4-Allyloxy-3,5-dimethyloxyphenylethylamine | Psychedelic and entactogen | ||||

|
Methallylescaline | OCH3 | OCH2C(CH2CH3) | OCH3 | 4-Methallyloxy-3,5-dimethoxyphenethylamine | Psychedelic and entactogen | ||||

|
Asymbescaline | OCH2CH3 | OCH2CH3 | OCH3 | 3,4-Diethoxy-5-methoxyphenethylamine | Psychedelic and euphoriant | ||||

|
DOM | CH3 | OCH3 | CH3 | OCH3 | 2,5-dimethoxy-4-methylamphetamine | Psychedelic | |||
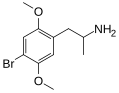
|
DOB | CH3 | OCH3 | Br | OCH3 | 2,5-dimethoxy-4-bromoamphetamine | Psychedelic | |||
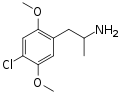
|
DOC | CH3 | OCH3 | Cl | OCH3 | 2,5-dimethoxy-4-chloroamphetamine | Psychedelic | |||
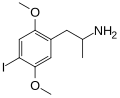
|
DOI | CH3 | OCH3 | I | OCH3 | 2,5-dimethoxy-4-iodoamphetamine | Psychedelic | |||
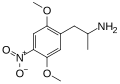
|
DON | CH3 | OCH3 | NO2 | OCH3 | 2,5-dimethoxy-4-nitroamphetamine | Stimulant | |||
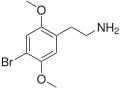
|
2C-B | OCH3 | Br | OCH3 | 2,5-dimethoxy-4-bromophenethylamine | Psychedelic, stimulant, entactogen and euphoriant | ||||
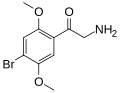
|
βk-2C-B | =O | OCH3 | Br | OCH3 | 2,5-dimethoxy-4-bromo-β-ketophenethylamine | Psychedelic, stimulant, entactogen and euphoriant | |||

|
2C-C | OCH3 | Cl | OCH3 | 2,5-dimethoxy-4-chlorophenethylamine | Psychedelic | ||||
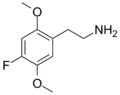
|
2C-F | OCH3 | F | OCH3 | 2,5-dimethoxy-4-fluorophenethylamine | Psychedelic | ||||

|
2C-I | OCH3 | I | OCH3 | 2,5-dimethoxy-4-iodophenethylamine | Psychedelic, stimulant | ||||

|
2C-D | OCH3 | CH3 | OCH3 | 2,5-dimethoxy-4-methylphenethylamine | Psychedelic, stimulant | ||||

|
2C-E | OCH3 | CH2-CH3 | OCH3 | 2,5-dimethoxy-4-ethylphenethylamine | Psychedelic | ||||
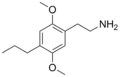
|
2C-P | OCH3 | CH2-CH3-CH3 | OCH3 | 2,5-dimethoxy-4-propylphenethylamine | Entactogen, euphoriant and Psychedelic | ||||
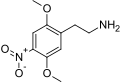
|
2C-N | OCH3 | NO2 | OCH3 | 2,5-dimethoxy-4-nitrophenethylamine | euphoriant | ||||
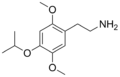
|
2C-O-4 | OCH3 | (CH3)2CHO | OCH3 | 2,5-Dimethoxy-4-propoxyphenethylamine | Hallucinogen, psychedelic and entheogenic[3] | ||||
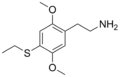
|
2C-T-2 | OCH3 | S-CH2CH3 | OCH3 | 2,5-dimethoxy-4-ethylthio-phenethylamine | Psychedelic | ||||
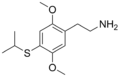
|
2C-T-4 | OCH3 | S-CH(CH3)2 | OCH3 | 2,5-dimethoxy-4-isopropylthio-phenethylamine | Psychedelic | ||||

|
2C-T-7 | OCH3 | S-CH2CH2CH3 | OCH3 | 2,5-dimethoxy-4-propylthio-phenethylamine | Psychedelic | ||||
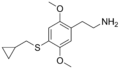
|
2C-T-8 | OCH3 | S-CH2-C3H5 | OCH3 | 2,5-dimethoxy-4-cyclopropylmethylthio-phenethylamine | Psychedelic | ||||

|
2C-T-19 | OCH3 | S-C(CH3)3 | OCH3 | 2,5-dimethoxy-4-tert-butylthio-phenethylamine | Psychedelic | ||||

|
2C-T-21 | OCH3 | S-CH2-CH2-F | OCH3 | 2,5-dimethoxy-4-(2-fluoroethylthio)-phenethylamine | Psychedelic and euphoriant | ||||

|
25B-NBOMe[4] | CH2-C6H4-OCH3 | OCH3 | Br | OCH3 | 2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||
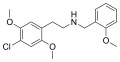
|
25C-NBOMe | CH2-C6H4-OCH3 | OCH3 | Cl | OCH3 | 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||
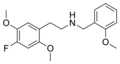
|
25F-NBOMe | CH2-C6H4-OCH3 | OCH3 | F | OCH3 | 2-(4-fluoro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||
|
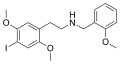
25I-NBOMe | CH2-C6H4-OCH3 | OCH3 | I | OCH3 | 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||

|
25D-NBOMe | CH2-C6H4-OCH3 | OCH3 | CH2 | OCH3 | 2-(4-methyl-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||

|
25E-NBOMe | CH2-C6H4-OCH3 | OCH3 | CH2-CH3 | OCH3 | 2-(4-ethyl-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||

|
25P-NBOMe | CH2-C6H4-OCH3 | OCH3 | CH2-CH3-CH3 | OCH3 | 2-(4-propyl-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine | Psychedelic | |||
|
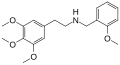
Mescaline-NBOMe | CH2-C6H4-OCH3 | OCH3 | OCH3 | OCH3 | N-(2-Methoxybenzyl)-2-(3,4,5-trimethoxyphenyl)ethanamine | Psychedelic | |||
|
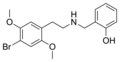
25B-NBOH | CH2–C6H4–OH | OCH3 | Br | OCH3 | N-(2-hydroxybenzyl)-2,5-dimethoxy-4-bromo-phenethylamine | Psychedelic | |||

|
25C-NBOH | CH2–C6H4–OH | OCH3 | Cl | OCH3 | N-(2-hydroxybenzyl)-2,5-dimethoxy-4-chloro-phenethylamine | Psychedelic | |||
|
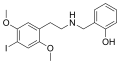
25I-NBOH | CH2–C6H4–OH | OCH3 | I | OCH3 | N-(2-hydroxybenzyl)-2,5-dimethoxy-4-iodo-phenethylamine | Psychedelic | |||
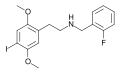
|
25I-NBF | CH2–C6H4–F | OCH3 | I | OCH3 | N-(2-fluorobenzyl)-2,5-dimethoxy-4-iodo-phenethylamine | Psychedelic | |||
| Short Name | RN | Rα | Rβ | R2 | R3 | R4 | R5 | Full Name | Biologic activity | |
Detection
[edit]This section needs expansion. You can help by adding to it. (December 2015) |
| Method | Requirement |
|---|---|
| UV spectrometry | Reagent needed |
Detection of substituted phenethylamines, which include compounds such as 2C-B, MDMA, and other designer drugs, involves various analytical methods aimed at identifying these psychoactive substances. These compounds are structurally similar to amphetamines, making their detection challenging due to potential cross-reactivity in standard drug tests. Techniques like gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), and immunoassay screenings are commonly employed for accurate identification. Advanced methods like high-performance liquid chromatography (HPLC) allow for precise separation and quantification of these substances even at low concentrations. Given the rising use of these drugs in recreational settings, developing sensitive and specific detection techniques remains crucial in forensic toxicology and clinical diagnostics.[citation needed]
See also
[edit]- Substituted amphetamine
- Substituted methylenedioxyphenethylamine
- Substituted cathinone
- Substituted phenylmorpholine
- 2Cs, DOx, 25-NB
- Substituted tryptamine
- PiHKAL
- The Shulgin Index
Notes
[edit]- ^ In other words, all of the compounds that belong to this class are structural analogs of phenethylamine.
- ^ Two ethyl groups attached to the amine group
References
[edit]- ^ Inan F, Brunt TM, Contrucci RR, Hondebrink L, Franssen EJ (April 2020). "Novel Phenethylamines and Their Potential Interactions With Prescription Drugs: A Systematic Critical Review". Therapeutic Drug Monitoring. 42 (2): 271–281. doi:10.1097/ftd.0000000000000725. PMID 32022784.
- ^ Wills B, Erickson T (2 February 2012). "Psychoactive Phenethylamine, Piperazine, and Pyrrolidinophenone Derivatives". Medical Toxicology of Drug Abuse. pp. 156–192. doi:10.1002/9781118105955.ch10. ISBN 978-0-471-72760-6.
- ^ Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. 2C-O-4 Entry in PiHKAL
- ^ Custodio RJ, Sayson LV, Botanas CJ, Abiero A, You KY, Kim M, et al. (November 2020). "25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential". Addiction Biology. 25 (6): e12850. doi:10.1111/adb.12850. PMID 31749223.
