Rhenium diselenide
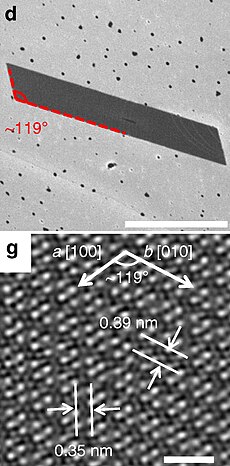 d: SEM micrograph of a typical parallelogram-shaped ReSe2 flake, scale bar 1 µm.
g: top view of the ReSe2 atomic lattice | |
 Unit cell of rhenium diselenide.
| |
| Names | |
|---|---|
| IUPAC name
Bis(selanylidene)rhenium
| |
| Other names
Rhenium(IV) selenide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.696 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| ReSe2 | |
| Molar mass | 344.13 g/mol[1] |
| Odor | odorless |
| Density | 9.22 g/cm3[2] |
| insoluble | |
| Band gap | ~1.2 eV (300 K), indirect[3] |
| Structure | |
| Triclinic, aP12, space group P1, No 2[2] | |
a = 0.6602 nm, b = 0.6716 nm, c = 0.6728 nm α = 91.82°, β = 104.9°, γ = 118.94°
| |
Formula units (Z)
|
4 |
| Related compounds | |
Other anions
|
Rhenium(IV) oxide Rhenium disulfide Rhenium ditelluride |
Other cations
|
Manganese diselenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rhenium diselenide is an inorganic compound with the formula ReSe2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
Synthesis
[edit]
Rhenium diselenide with a thickness as small as a triple-atomic layer can be produced by chemical vapor deposition at ambient pressure. A mixture of Ar and hydrogen gases is flown through a tube whose ends are kept at different temperatures. The substrate and ReO3 powder are placed at the hot end which is heated to 750 °C, and selenium powder is located at the cold end which is kept at 250 °C.[4]
- 2 ReO3 + 7 Se → 2 ReSe2 + 3 SeO2
Properties
[edit]
As most other dichalcogenides of transition metals, rhenium diselenide has a layered structure where atoms are strongly bonded within each layer and the layers are held together by weak Van der Waals bonds. However, while most other layered dichalcogenides have a high (hexagonal) symmetry, ReSe2 has a very low triclinic symmetry, and this symmetry does not change from the bulk to monolayers.[4]
References
[edit]- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.84. ISBN 1-4398-5511-0.
- ^ a b Wildervanck, J.C; Jellinek, F (1971). "The dichalcogenides of technetium and rhenium". Journal of the Less Common Metals. 24: 73–81. doi:10.1016/0022-5088(71)90168-8.
- ^ Dumcenco, D; Huang, Y. S; Liang, C. H; Tiong, K. K (2008). "Optical characterization of Au-doped rhenium diselenide single crystals". Journal of Applied Physics. 104 (6): 063501–063501–6. Bibcode:2008JAP...104f3501D. doi:10.1063/1.2977682.
- ^ a b Jiang, Shaolong; Hong, Min; Wei, Wei; Zhao, Liyun; Zhang, Na; Zhang, Zhepeng; Yang, Pengfei; Gao, Nan; Zhou, Xiebo; Xie, Chunyu; Shi, Jianping; Huan, Yahuan; Tong, Lianming; Zhao, Jijun; Zhang, Qing; Fu, Qiang; Zhang, Yanfeng (2018). "Direct synthesis and in situ characterization of monolayer parallelogrammic rhenium diselenide on gold foil". Communications Chemistry. 1. doi:10.1038/s42004-018-0010-6.
