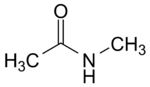
N-Methylacetamide
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-Methylacetamide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.075 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 85335 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H7NO | |
| Molar mass | 73.095 g·mol−1 |
| Appearance | Colourless solid with faint odour[1] |
| Density | 0.94 g·cm−3[1] |
| Melting point | 27–30.6 °C (80.6–87.1 °F; 300.1–303.8 K)[1][4] |
| Boiling point | 206–208 °C (403–406 °F; 479–481 K)[1] |
| Solubility | |
| Vapor pressure | 1.1 hPa (50 °C)[1] |
Refractive index (nD)
|
1.433 (20 °C)[3] |
| Hazards | |
| GHS labelling:[1] | |

| |
| Danger | |
| H360D | |
| P201, P202, P280, P308+P313, P405, P501[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
N-Methylacetamide is a chemical compound from the amides group. The compound is listed as very high concern by the European Chemicals Agency (ECHA).
Generation
[edit]N-methylacetamide can be obtained by reacting hot acetic acid or acetic anhydride with methylamine.[5][6] It can also be prepared by reacting N,N′-dimethylurea with acetic acid[6] or by reacting acetone oxime with sulfuric acid.[7]
Characteristics
[edit]N-Methylacetamide is a flammable, difficult to ignite, hygroscopic, crystalline, colourless solid with a faint odor that is soluble in water.[1] Several isomeric forms are known.[8][9] In solution, it is 97–100% present as the Z isomer with a polymeric structure.[10][4] The compound has a high dielectric constant of 191.3 at 32 °C.[11]
Use
[edit]N-Methylacetamide is used as an intermediate in the production of agrochemicals and as a solvent in electrochemistry.[2][5]
See Also
[edit]References
[edit]- ^ a b c d e f g h i Record of N-Methylacetamid in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2022-01-20.
- ^ a b N-Methylacetamide, 99% at AlfaAesar, accessed on {{{Datum}}} (PDF) (JavaScript required).[dead link]
- ^ Sigma-Aldrich Co., product no. {{{id}}}.
- ^ a b J. F. Coetzee (2013). Recommended Methods for Purification of Solvents and Tests for Impurities International Union of Pure and Applied Chemistry. Elsevier. p. 50. ISBN 978-1-4831-3845-9.
- ^ a b NLM Hazardous Substances Data Bank entry for [ ]
- ^ a b D.R. Buhler, D.J. Reed (2013). Nitrogen and Phosphorus Solvents. Elsevier. p. 166. ISBN 978-1-4832-9020-1.
- ^ Joachim Buddrus, Bernd Schmidt (2015). Grundlagen der Organischen Chemie. Walter de Gruyter GmbH & Co KG. p. 581. ISBN 978-3-11-033105-9.
- ^ Noemi G. Mirkin, Samuel Krimm (1991). "Conformers of cis-N-methylacetamide". Journal of Molecular Structure: THEOCHEM. 236 (1–2): 97–111. doi:10.1016/0166-1280(91)87010-J. hdl:2027.42/29043.
- ^ Mirkin, Noemi G.; Krimm, Samuel (1991-12-01). "Ab initio vibrational analysis of hydrogen-bonded trans- and cis-N-methylacetamide". Journal of the American Chemical Society. 113 (26): 9742–9747. Bibcode:1991JAChS.113.9742M. doi:10.1021/ja00026a005.
- ^ K.-H. Hellwich (2013). Stereochemie Grundbegriffe. Springer-Verlag. p. 43. ISBN 978-3-662-10051-6.
- ^ A. Covington (2012). Physical Chemistry of Organic Solvent Systems. Springer Science & Business Media. p. 247. ISBN 978-1-4684-1959-7.
