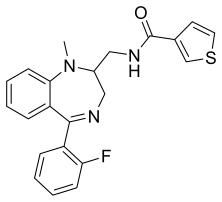
Tifluadom
 | |
| Clinical data | |
|---|---|
| Routes of administration | unknown |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.073.052 |
| Chemical and physical data | |
| Formula | C22H20FN3OS |
| Molar mass | 393.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tifluadom is a benzodiazepine derivative with an unusual activity profile.[1] Unlike most benzodiazepines, tifluadom has no activity at the GABAA receptor, but instead is a selective agonist for the κ-opioid receptor.[2] It has potent analgesic[3] and diuretic[4] effects in animals, and also has sedative effects and stimulates appetite.[5][6]
While tifluadom has several effects which might have potential uses in medicine, such as analgesia and appetite stimulation, κ-opioid agonists tend to produce undesirable effects in humans such as dysphoria and hallucinations, and so these drugs tend to only be used in scientific research. Dysphoric effects are similar to those seen when using other κ-opioid receptor agonists like pentazocine and salvinorin A, and can be considered the opposite of morphine-induced euphoria. As such, kappa agonists are believed to have very limited abuse potential.
See also
[edit]- Lufuradom
- GYKI-52895, a benzodiazepine which is a dopamine reuptake inhibitor without GABAergic function
- GYKI-52,466, a benzodiazepine which is an AMPAkine and glutamate antagonist without GABAergic function
References
[edit]- ^ US 4325957, Zeugner H, Roemer D, Liepmann H, Milkowski W, "2-Acylaminomethyl-1,4-benzodiazepine derivatives and their salts and pharmaceutical compositions thereof", issued 20 April 1982, assigned to Abbott Products GmbH
- ^ Römer D, Büscher HH, Hill RC, Maurer R, Petcher TJ, Zeugner H, Benson W, Finner E, Milkowski W, Thies PW (1982). "Unexpected opioid activity in a known class of drug". Life Sciences. 31 (12–13): 1217–20. doi:10.1016/0024-3205(82)90346-0. PMID 6292610.
- ^ Genovese RF, Dykstra LA (November 1986). "Tifluadom's effects under electric shock titration and tail-immersion procedures in squirrel monkeys". Life Sciences. 39 (19): 1713–9. doi:10.1016/0024-3205(86)90089-5. PMID 3773641.
- ^ Leander JD (March 1984). "Kappa opioid agonists and antagonists: effects on drinking and urinary output". Appetite. 5 (1): 7–14. doi:10.1016/s0195-6663(84)80044-6. PMID 6091543. S2CID 31380360.
- ^ Jackson HC, Sewell RD (October 1984). "The role of opioid receptor sub-types in tifluadom-induced feeding". The Journal of Pharmacy and Pharmacology. 36 (10): 683–6. doi:10.1111/j.2042-7158.1984.tb04843.x. PMID 6150086. S2CID 13046110.
- ^ Dykstra LA, Gmerek DE, Winger GA, Woods JH (August 1987). "Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects". Journal of Pharmacology and Experimental Therapeutics. 242 (2): 413–20. PMID 3612543.
