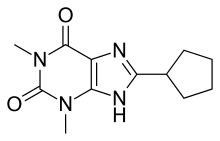
8-Cyclopentyl-1,3-dimethylxanthine
Appearance
From Wikipedia, the free encyclopedia
(Redirected from C12H16N4O2)
Chemical compound
Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H16N4O2 |
| Molar mass | 248.286 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
8-Cyclopentyl-1,3-dimethylxanthine (8-Cyclopentyltheophylline, 8-CPT, CPX) is a drug which acts as a potent and selective antagonist for the adenosine receptors, with some selectivity for the A1 receptor subtype, as well as a non-selective phosphodiesterase inhibitor. It has stimulant effects in animals with slightly higher potency than caffeine.[1][2]
See also
[edit]References
[edit]- ^ Spealman RD (1988). "Psychomotor stimulant effects of methylxanthines in squirrel monkeys: relation to adenosine antagonism". Psychopharmacology. 95 (1): 19–24. doi:10.1007/bf00212759. PMID 3133696. S2CID 11539292.
- ^ Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, et al. (July 2003). "Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration". Neuropsychopharmacology. 28 (7): 1281–91. doi:10.1038/sj.npp.1300167. PMID 12700682.
| PDE1 | |
|---|---|
| PDE2 | |
| PDE3 | |
| PDE4 |
|
| PDE5 | |
| PDE7 | |
| PDE9 | |
| PDE10 | |
| PDE11 | |
| Non-selective | |
| Unsorted | |
See also: Receptor/signaling modulators | |
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
