Barium iodide
| |
| Names | |
|---|---|
| IUPAC name
Barium iodide
| |
| Other names
Barium iodide, anhydrous
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.873 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaI2 (anhydrous) BaI2·2H2O (dihydrate) | |
| Molar mass | 391.136 g/mol (anhydrous) 427.167 g/mol (dihydrate) |
| Appearance | White orthorhombic crystals (anhydrous) colorless crystals (dihydrate) |
| Odor | odorless |
| Density | 5.15 g/cm3 (anhydrous) 4.916 g/cm3 (dihydrate) |
| Melting point | 711 °C (1,312 °F; 984 K) (anhydrous) decomposes at 740 °C (dihydrate) |
| 166.7 g/100 mL (0 °C) 221 g/100 mL (20 °C) 246.6 g/100 mL (70 °C) | |
| Solubility | soluble in ethanol, acetone |
| -124.0·10−6 cm3/mol | |
| Structure | |
| PbCl2-type (Orthorhombic oP12) | |
| Pnma (No. 62) | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-602.1 kJ·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| Related compounds | |
Other anions
|
barium fluoride barium chloride barium bromide |
Other cations
|
beryllium iodide magnesium iodide calcium iodide strontium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium iodide is an inorganic compound with the formula BaI2. The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids. When heated, hydrated barium iodide converts to the anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
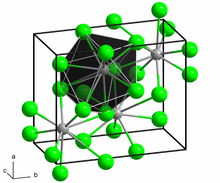
Structure
[edit]The structure of the anhydrous form resembles that of lead(II) chloride with each Ba center bound to nine iodide ligands[2] and has a crystalline packing structure that is quite similar to BaCl2.[3]
Reactions
[edit]Anhydrous BaI2 can be prepared by treating Ba metal with 1,2-diiodoethane in ether.[4]
BaI2 reacts with alkyl potassium compounds to form organobarium compounds.[5]
BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.[6]
Safety
[edit]Like other soluble salts of barium, barium iodide is toxic.
References
[edit]- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–44, ISBN 0-8493-0594-2
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Brackett, E. B.; Brackett, T. E.; Sass, R. L.; The Crystal Structures of Barium Chloride, Barium Bromide, and Barium Iodide. J. Phys. Chem., 1963, volume 67, 2132 – 2135
- ^ Duval, E.; Zoltobroda, G.; Langlois, Y.; A new preparation of BaI2: application to (Z)-enol ether synthesis. Tetrahedron Letters, 2000, 41, 337-339
- ^ Walter, M. D.; Wolmershauser, G.; Sitzmann, H.; Calcium, Strontium, Barium, and Ytterbium Complexes with Cyclooctatetraenyl or Cyclononatetraenyl Ligands. J. Am. Chem. Soc., 2005, 127 (49), 17494 – 17503.
- ^ Yanagisawa, A.; Habaue, S.; Yasue, K.; Yamamoto, H.; Allylbarium Reagents: Unprecedented Regio- and Stereoselective Allylation Reactions of Carbonyl Compounds. J. Am. Chem. Soc.1994, 116,6130-6141
