2-Coumaranone
 | |
| Names | |
|---|---|
| IUPAC name
3H-1-benzofuran-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.230 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H6O2 | |
| Melting point | 49–51 °C[1] |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H315, H317, H319 | |
| P261, P264, P264+P265, P272, P280, P302+P352, P305+P351+P338, P321, P332+P317, P333+P317, P337+P317, P362+P364, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Coumaranone (sometimes also called 2-Cumaranone[3]) is a bicyclic heteroaromatic compound in which a six-membered benzene ring is annulated with a five-membered γ-butyrolactone ring. The 2(3H)-benzofuranone can also be considered as a lactone of (2-hydroxyphenyl)acetic acid. The benzofuranone basic structure is the basis of some natural products – such as rosmadial,[4] which is isolatable from rosemary oil, and some substances with high pharmacological activity, such as griseofulvin and rifampicin. Furthermore, 2-cumaranone is utilized as a starting material for the preparation of chemiluminescent and fluorescent dyes, for synthetic pharmaceutical agents, like the antiarrhythmic drug dronedarone, and especially for the fungicide azoxystrobin.
Occurrence and synthesis
[edit]In 1884, Adolf von Baeyer and Paul Fritsch disclosed the synthesis of 2-coumaranone, which they described as the lactone of o-oxyphenylacetic acid, through the distillation "over free fire" of (2-hydroxyphenyl)acetic acid.[5]

The lactone 3H-benzofuran-2-one forms in this process under intramolecular water splitting at high temperature and in an impure state.
A similar fragmentation by oxidative intramolecular ring closure from phenylacetic acid also yields only modest returns (< 20%) due to the oxidation sensitivity of the methylene group and the formation of several by-products 2-coumaranone.[6]

The Ozonolysis of 2-allylphenol obtained by the alkylation of phenol with 3-bromopropene to produce phenylallyl ether and subsequently its Claisen rearrangement, gives rise to 2-hydroxyphenylacetic acid, which, through water splitting, yields 2-coumaranone. Despite this method having good yields, its economic and safety considerations make it unsuitable for an industrial process.[7]

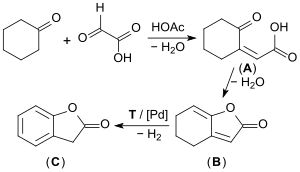
On an industrial scale, the well-filtered starting materials cyclohexanone and glyoxylic acid are first transformed in an acid-catalyzed aldol condensation to form the (predominantly) cis-2-oxocyclohexylidene acetic acid (A). This then, in a second step, is transformed into the so-called enollactone (B) through water elimination (90% yield). The enollactone is continuously dehydrogenated at 250 °C in the vapor phase on a palladium catalyst to form 2-coumaranone (C) dehydrogenation (yield approximately 67%).[8][9][10]
An alternative process that uses glyoxylic acid methyl ester methylhemiacetals rather than glyoxylic acid as a starting material has not gained widespread acceptance.[11]
Properties
[edit]Pure 2-coumaranone manifests as an off-white to pale yellow solid with an aromatic odor.[1] On purification by distillation, "a colorless oil passes which solidifies in the receiver into splendid, transparent, well-formed crystals".[12] 3H-benzofuran-2-one is soluble in hot water, diethyl ether[5] and acetonitrile.[11] The lactone hydrolyzes slowly in hot water and rapidly in aqueous alkalis to form 2-hydroxyphenylacetic acid or its alkali salt.[5]
Applications
[edit]5-Nitro-3H-benzofuran-2-one is formed during the nitration of 2-coumaranone with nitrating acid.[13][14]

The 5-amino-3H-benzofuran-2-one can be obtained from the nitro compound using catalytic hydrogenation at a palladium catalyst.[13]
Lactones such as 2-coumaranone ('I) are readily cleaved by nucleophiles, leading to ring opening. Thus, 5-nitro-3H-benzofuran-2-one reacts with secondary amines to form 2-hydroxyphenylacetic acid amides. Through hydrogenation, these transform into corresponding 3-amino-6-hydroxyphenylethylamines, which are useful precursors for hair dyeing.[14]

Condensation of 5-nitro-3H-benzofuran-2-one (II) with a mixture of valeric acid (III) and valeric anhydride (IV) results in the enollactone (V), which upon heating rearranges to the substituted benzofurancarboxylic acid (VI), a key precursor for the antiarrhythmic drug Dronedarone.[15]

The basic structure of 2-coumaranone also underlies a class of antioxidants and radical scavengers, especially for stabilizing polypropylenes. In the synthesis of a model compound, glyoxylic acid reacts with 2 moles of 4-tert-butylphenol in the presence of methanesulfonic acid CH3SO3H to form a phenolic intermediate and is then esterified with benzoic acid.[16]

A one-pot reaction, carried out as a Tscherniak-Einhorn reaction of fluorophenols (X), glyoxylic acid (Y), and carbamates, such as carbamic acid methyl ester[17] or carbamic acid mesityl ester (Z), yields 2-coumaranones with carbamide side chains. These compounds react with strong bases such as diazabicycloundecene or potassium tert-butanolate, and show pronounced chemiluminescence in the presence of oxygen.[18][19][20]

The most notable application of 2-coumaranone by volume is as a starting material for the synthesis of the fungicide azoxystrobin[21] (known as Amistar from Syngenta) in the class of strobilurins.
References
[edit]- ^ a b Sigma-Aldrich Co., product no. {{{id}}}.
- ^ "2-Coumaranone". pubchem.ncbi.nlm.nih.gov.
- ^ PubChem. "2-Coumaranone". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-01-16.
- ^ N. Nakatani; R. Inatani (1983), "A New Diterpene Lactone, Rosmadial, from Rosemary (Rosmarinus officinalis L.)", Biosci. Biotechnol. Biochem., vol. 47, no. 2, pp. 353–358, doi:10.1080/00021369.1983.10865620
- ^ a b c A. Baeyer; P. Fritsch (1884), "Ueber die o-Oxyphenylessigsäure und ihre Derivate", Chem. Ber., vol. 17, no. 1, pp. 973–975, doi:10.1002/cber.188401701258
- ^ T. Fukagawa; Y. Fujiwara; H. Taniguchi (1982), "Palladium-catalyzed intramolecular aromatic nuclear acyloxylation: preparation of 2-coumaranone", J. Org. Chem., vol. 47, no. 12, pp. 2491–2493, doi:10.1021/jo00133a055
- ^ EP 1481959, W. Jary, "Verfahren zur Herstellung von Lactonen und von aromatischen Hydroxycarbonsäuren", published 2004-12-01, assigned to DSM Fine Chemicals Austria Nfg GmbH & CO., KG
- ^ N. Carmona; P. Gallezot; A. Perrard; L. Carmona; G. Mattioda; J.-C. Vallejos (1998), Synthesis of 2-Coumaranone by Catalytic Dehydrogenation of α-Carboxymethylidene Cyclohexanone, in Catalysis of Organic Reactions, Frank E. Herkes, editor, New York, NY, U.S.A.: Marcel Dekker, Inc., pp. 381–390, ISBN 0-8247-1929-8
- ^ US 5616733, J.-C. Vallejos; A. Perrard & Y. Christidis et al., "Preparation method for 2-coumaranone", published 1997-4-1, assigned to Société Française Hoechst
- ^ EP 0818451, N. Carmona; L. Carmona & A. Perrard et al., "Procédé de préparation de l'énollactone de l'acide 2-oxocyclohexylidène acétique et application à la préparation de la 2-coumaranone", published 1998-01-14, assigned to Clariant Chimie S.A.
- ^ a b EP 149838, M. Stanek; P. Hildebrand & C. Zimmermann et al., "Verfahren zur Herstellung von 2-Coumaron und substituierten 2-Coumaronen", published 2004-08-25, assigned to DSM Fine Chemicals Austria Nfg GmbH & CO., KG
- ^ S. Czaplicki; Stanislaus von Kostanecki; V. Lampe (1909), "Versuche zur Synthese des Chromenols und seiner Derivate", Chem. Ber., vol. 42, no. 1, pp. 827–838, doi:10.1002/cber.190904201133
- ^ a b Christopher E. Malmberg (2015). "Total Synthesis of Clavatadine A Analogs to Produce a Viable Reversible Inhibitor for Factor XIa" (PDF). MS Thesis. Central Washington University Central Washington University. p. 14. Retrieved 2022-06-20.
- ^ a b US 7070630, M.-I. Lim, Y.-G. Pan, "Primary intermediates für oxidative coloration of hair", published 2006-4-7, assigned to The Procter & Gamble Co.
- ^ EP 2508517, A. Shoutteeten, F. Bleger, F. Mordacq, J. Piron, "Process for the preparation of N-alkyl-2(hydroxy-4-benzoyl)-3-benzofurans and its intermediates thereof", published 2012-10-10, assigned to Clariant Specialty Fine Chemicals (France)
- ^ EP 2500341, C.-F. Chiu, C.-Y. Su, S. Lee, "Benzofuranone derivatives and application of the same", published 2013-06-26, assigned to Chitec Technology Co., Ltd., Double Bond Chemical Ind., Co., Ltd., FDC, Lees Chemical Industry Co. Ltd.
- ^ R. Krieg; B. Hoffmann; D. Weiß; C. Biskup (2019), "First synthesis of highly chemiluminescent benzo[b]furan-2(3H)-ones bearing a urea substructure", Helv. Chim. Acta, 102 (6): e1800243, doi:10.1002/hlca.201800243, S2CID 107893512
- ^ S. Schramm; et al. (2013), "Investigations on the synthesis and chemiluminescence of novel 2-coumaranones", Arkivoc, vol. 3, pp. 174–188
- ^ S. Schramm; et al. (2015), "Investigations on the synthesis and chemiluminescence of novel 2-coumaranones – II", Arkivoc, vol. 5, pp. 44–59
- ^ "2-Coumaranone-1-L" (PDF; 170 kB). caymanchem.com. Cayman Chemical Co. Retrieved 2022-06-20.
- ^ WO 199208703, J.D. Jones, G.A. DeBoos, P. Wilkinson, B.G. Cox, J.M. Fielden, "Process for the preparation of pyrimidine compounds", published 1992-5-29, assigned to Imperial Chemical Industries PLC
