Methanesulfonic acid
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanesulfonic acid | |
| Other names
Methylsulfonic acid, MSA; Mesylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1446024 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.817 |
| EC Number |
|
| 1681 | |
PubChem CID
|
|
| UNII | |
| UN number | 2585 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH4O3S | |
| Molar mass | 96.10 g·mol−1 |
| Appearance | Clear, colourless liquid |
| Density | 1.48 g/cm3 |
| Melting point | 17 to 19 °C (63 to 66 °F; 290 to 292 K) |
| Boiling point | 167 °C (333 °F; 440 K) at 10 mmHg, 122 °C/1 mmHg |
| miscible | |
| Solubility | Miscible with methanol, diethyl ether. Immiscible with hexane |
| log P | −2.424[1] |
| Acidity (pKa) | −1.9[2] |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
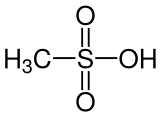
Methanesulfonic acid (MsOH, MSA) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the molecular formula CH3SO3H and structure H3C−S(=O)2−OH. It is the simplest of the alkylsulfonic acids (R−S(=O)2−OH). Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric acid (HCl) or sulfuric acid (H2SO4).[3]
History and synthesis
[edit]Early history
[edit]German chemist Hermann Kolbe discovered MSA between 1842 and 1845 and originally termed it methyl hyposulphuric acid.[4][5][6]
The discovery stemmed from earlier work by Berzelius and Marcet in 1813, who treated carbon disulfide with moist chlorine and produced a compound they named "sulphite of chloride of carbon". By reacting it with barium hydroxide Kolbe demonstrated it to actually be trichloromethylsulfonyl chloride (CCl₃SO₂Cl in modern notation).[4][5]
2 CCl3SO2Cl + 3 Ba(OH)2 → Ba(CCl3SO3)2 + 3 BaCl2 + 2 H2O
From resulting barium trichloromethylsulfonate Kolbe isolated the free acid, which he was then able to sequentially dechlorinate by electrolytically generated atomic hydrogen to ultimately yield MSA.[4][5]
CCl3SO3H + 3 H → CHCl2SO3H + 2 H + HCl → … → CH3SO3H + 3 HCl
Kolbe's research on methanesulfonic and chloroacetic acids was hailed by Berzelius as strong evidence for his theory of copulated compounds, a modification of radical theory to accommodate substitution reactions which posited the combination of organic and inorganic moieties without significantly altering the properties of the latter.[6]
Later in the 19th century, the name transitioned to methyl sulphonic acid. Other historical laboratory synthesis routes included oxidizing methanethiol, dimethyl disulfide or methyl thiocyanate with nitric acid.[5]
Industrial production
[edit]The first commercial production of MSA, developed in the 1940s by Standard Oil of Indiana, was based on oxidation of dimethylsulfide by O
2 from air. Although inexpensive, this process suffered from a poor product quality and explosion hazards.
Starting from the 1960s, it received a shortened name of mesylic acid.[7]
In 1967, the Pennwalt Corporation (USA) developed a different process for dimethylsulfide (as a water-based emulsion) oxidation using chlorine, followed by extraction-purification. In 2022 this chlorine-oxidation process was used only by Arkema (France) for making high-purity MSA. This process is not popular on a large scale, because it co-produces large quantities of hydrochloric acid.
Between years 1970 and 2000 MSA was used only on a relatively small-scale in niche markets (for example, in the microelectronic and electroplating industries since the 1980s), which was mainly due to its rather high price and limited availability. However, this situation changed around 2003, when BASF launched commercial production of MSA in Ludwigshafen based on a modified version of the aforementioned air oxidation process, oxidising dimethyldisulfide with nitric acid which is then restored using atmospheric oxygen. The former is produced in one step from methanol from syngas, hydrogen and sulfur.[8]
An even better (lower-cost and environmentally friendlier) process of making methanesulfonic acid was developed in 2016 by Grillo-Werke AG (Germany). It is based on a direct reaction between methane and oleum at around 50 °C and 100 bar in the presence of a potassium persulfate initiator. [9] Further addition of sulfur trioxide gives methanedisulfonic acid instead.[10] This technology was acquired and commercialized by BASF in 2019.[11]
Applications
[edit]Since ca. 2000 methanesulfonic acid has become a popular replacement for other acids in numerous industrial and laboratory applications, because it:
- is a strong acid,
- has a low vapor pressure (see boiling points in the "Properties" inset),
- is not an oxidant or explosive, like nitric, sulfuric or perchloric acids.
- is a liquid at room temperature,
- is soluble in many organic solvents,
- forms water-soluble salts with all inorganic cations and with most organic cations,
- does not form complexes with metal ions in water,
- its anion, mesylate, is non-toxic and suitable for pharmaceutical preparations.
The closely related p-toluenesulfonic acid (PTSA) is solid.
Methanesulfonic acid can be used in the generation of borane (BH3) by reacting methanesulfonic acid with NaBH4 in an aprotic solvent such as THF or DMSO, the complex of BH3 and the solvent is formed.[12]
Applications
[edit]Solutions of methanesulfonic acid are used for the electroplating of tin and tin-lead solders. It is displacing the use of fluoroboric acid, which releases corrosive and volatile hydrogen fluoride.[13]
Methanesulfonic acid is also a primary ingredient in rust and scale removers.[14] It is used to clean off surface rust from ceramic, tiles and porcelain which are usually susceptible to acid attack.
See also
[edit]- Trifluoromethanesulfonic acid, the more acidic trifluoro analogue
References
[edit]- ^ Towler, Christopher S.; Li, Tonglei; Wikström, Håkan; Remick, David M.; Sanchez-Felix, Manuel V.; Taylor, Lynne S. (December 2008). "An Investigation into the Influence of Counterion on the Properties of Some Amorphous Organic Salts". Molecular Pharmaceutics. 5 (6): 946–955. doi:10.1021/mp8000342. PMID 19434850.
- ^ Guthrie, J. Peter (September 1978). "Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units". Canadian Journal of Chemistry. 56 (17): 2342–2354. doi:10.1139/v78-385.
- ^ Gernon, M. D.; Wu, M.; Buszta, T.; Janney, P. (1999). "Environmental benefits of methanesulfonic acid: comparative properties and advantages". Green Chemistry. 1 (3): 127–140. doi:10.1039/a900157c.
- ^ a b c Kolbe, H. (January 1845). "Beiträge zur Kenntniss der gepaarten Verbindungen". Justus Liebigs Annalen der Chemie. 54 (2): 145–188. doi:10.1002/jlac.18450540202. ISSN 0075-4617.
- ^ a b c d Roscoe, Henry Enfield (1890). A Treatise on Chemistry: The chemistry of the hydrocarbons and their derivatives, or Organic chemistry. D. Appleton and Company. p. 215.
- ^ a b Rocke, Alan J. (1993-01-01). The Quiet Revolution: Hermann Kolbe and the Science of Organic Chemistry. University of California Press. p. 59. ISBN 978-0-520-08110-9.
- ^ Marler, E. E. J. (1967). Pharmacological and Chemical Synonyms: A Collection of Names of Drugs and Other Compounds Drawn from the Medical Literature of the World. Excerpta Medica.
- ^ US patent 6531629B1, Matthias Eiermann, Christian Tragut, Klaus Ebel, "Method of producing alkanesulfonic acid", issued 2003-03-11, assigned to BASF SE
- ^ Lobree, Lisa J.; Bell, Alexis T. (2001). "K2S2O8-Initiated Sulfonation of Methane to Methanesulfonic Acid". Ind. Eng. Chem. Res. 40 (3): 736–742. doi:10.1021/ie000725b.
- ^ Kosswig, Kurt (2000). "Sulfonic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_503. ISBN 3527306730.
- ^ Binnemans, K.; Jones, P. T. (2022). "Methanesulfonic Acid (MSA) in Hydrometallurgy". Journal of Sustainable Metallurgy. 20: 26–45. doi:10.1007/s40831-022-00641-6.
- ^ Lobben, Paul C.; Leung, Simon Shun-Wang; Tummala, Srinivas (2004). "Integrated Approach to the Development and Understanding of the Borane Reduction of a Carboxylic Acid". Org. Process Res. Dev. 8 (6): 1072–1075. doi:10.1021/op049910h.
- ^ Balaji, R.; Pushpavanam, Malathy (2003). "Methanesulphonic acid in electroplating related metal finishing industries". Transactions of the Imf. 81 (5): 154–158. doi:10.1080/00202967.2003.11871526. S2CID 91584456.
- ^ "Safety Data Sheet" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2015-12-01.
