Indoprofen
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High (rapid and complete absorption) |
| Metabolism | Glucuronidation |
| Elimination half-life | 2.3 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.197 |
| Chemical and physical data | |
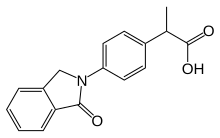
| Formula | C17H15NO3 |
| Molar mass | 281.311 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Indoprofen is a nonsteroidal anti-inflammatory drug (NSAID). It was withdrawn worldwide in the 1980s after postmarketing reports of severe gastrointestinal bleeding.[1]
A 2004 study using high-throughput screening found indoprofen to increase production of the survival of motor neuron protein, suggesting it may provide insight into treatments for spinal muscular atrophies.[1][2]
Synthesis
[edit]The isoindolone ring system forms the nucleus for this profen NSAID.
The nitro group in 2-(4-nitrophenyl)propionic acid (1) is reduced using iron and hydrochloric acid to give 2-(4-aminophenyl)propionic acid (2). Reaction with phthalic anhydride then gives the phthalimide (4). Treatment with zinc in acetic acid yields indoprofen after reduction of one of the amide groups.[3][4][5]
See also
[edit]References
[edit]- ^ a b Frazin N (March 9, 2005). "Pain Reliever May Provide Clues for Treating Spinal Muscular Atrophy". United States National Institute of Neurological Disorders and Stroke. Archived from the original on 2008-07-04. Retrieved 2007-10-06.
- ^ Lunn MR, Root DE, Martino AM, Flaherty SP, Kelley BP, Coovert DD, et al. (November 2004). "Indoprofen upregulates the survival motor neuron protein through a cyclooxygenase-independent mechanism". Chemistry & Biology. 11 (11): 1489–93. doi:10.1016/j.chembiol.2004.08.024. PMC 3160629. PMID 15555999.
- ^ US patent 4316850, Richard W. J. Carney and George de Stevens, "Tertiary aminoacids", issued 1982-02-23, assigned to Ciba Geigy Corp
- ^ Nannini G, Giraldi PN, Molgora G, Biasoli G, Spinelli F, Logemann W, et al. (August 1973). "New analgesic-anti-inflammatory drugs. 1-Oxo-2-substituted isoindoline derivatives". Arzneimittel-Forschung. 23 (8): 1090–100. doi:10.1002/chin.197344288. PMID 4801034.
- ^ "Indoprofen". Pharmaceutical Substances. Thieme. Retrieved 2024-07-11.

