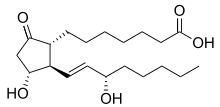
Prostaglandin E1
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Caverject, Muse, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695022 |
| License data |
|
| Routes of administration | Intravenous, intracavernous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.925 |
| Chemical and physical data | |
| Formula | C20H34O5 |
| Molar mass | 354.487 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Prostaglandin E1 (PGE1) is a naturally occurring prostaglandin with various medical uses. Alprostadil and misoprostol are synthetic forms of prostaglandin E1 used as medications. Lubiprostone, a derivative of prostaglandin E1, is also used as a medication.[2][3] Prostaglandin E1 is a vasodilator. It has various effects in the body that include opening blood vessels, relaxing smooth muscle, inhibiting clotting, and causing uterine contractions.[2][4]
In infants with certain congenital heart defects, alprostadil is delivered by slow injection into a vein to maintain a patent ductus arteriosus until surgery can be carried out.[4] By injection into the penis or placement in the urethra, alprostadil is used to treat erectile dysfunction.[5] Common side effects when given to babies include decreased breathing, fever, and low blood pressure.[2] When injected into the penis for erectile dysfunction; side effects may include penile pain, bleeding at the site of injection, and prolonged erection (priapism).[2] Prostaglandin E1 was isolated in 1957 and approved for medical use in the United States in 1981.[2][6]
Misoprostol has various obstetric uses. It is used to induce abortion, to completely empty the uterus after a miscarriage, to induce labor, and to prevent and treat postpartum hemorrhage. The medication is available through many routes. It can be swallowed, dissolved in the mouth, placed in the vagina, or placed in the rectum. Misoprostol can also be used to manage duodenal ulcers and peptic ulcer disease when other medications are not effective.[7] It is on the World Health Organization's List of Essential Medicines for its obstetric uses.[8]
Lubiprostone is a PGE1 derivative used to treat chronic constipation. It is taken orally.[9] Common side effects include diarrhea, vomiting, and abdominal pain.[10]
Biosynthesis
[edit]Prostaglandin E1 is biosynthesized on an as-needed basis from dihomo-γ-linolenic acid (an omega-6 fatty acid) in healthy humans without coronary artery disease[11] and/or a genetic disorder.
Medical uses
[edit]Alprostadil
[edit]Alprostadil is used for two main purposes: the treatment of newborns with congenital heart defects and in treatment of erectile dysfunction.[3]
Some babies are born with heart defects which compromise blood flow to the body. In some defects, including tetralogy of Fallot, aortic valvular atresia, and Eisenmenger pulmonary hypertension, alprostadil is given intravenously by a nurse until surgery can be performed to repair the defect.[7] The medication maintains a patent ductus arteriosus. The ductus arteriosus is a shortcut between the aorta and pulmonary artery, two large vessels branching off the heart. This shortcut allows the fetus to maintain blood flow before birth. However, it closes shortly after birth when the newborn can breathe oxygen. In babies with certain congenital defects, however, it's essential to keep the ductus arteriosus open to ensure at least some blood flow throughout the body. Side effects include decreased breathing or a lack of breathing at high doses. The medication can also cause hypotension, or low blood pressure.[4]
Alprostadil is also used for adults with erectile dysfunction. It is typically given when other medications are ineffective. The medication relaxes smooth muscle and allows the arteries in the corpus cavernosum of the penis to dilate. Blood then flows to the penis. This blood flow compresses the veins which drain blood from the penis, trapping the blood in the corpus cavernosum. This process leads to erection.[12] When injected into the base of the penis, alprostadil may cause penile pain, dizziness, headache, high blood pressure, Peyronie disease, and burning at the injection site. When inserted into the urethra, alprostadil may cause urethral burning, penile pain, dizziness, headache, and urethral bleeding. When someone engages in vaginal sex after using an alprostadil urethral suppository, their partner may experience vaginal itching.[3]
Clinical trials for the treatment showed positive results in around 3,000 men that it was tested on; it is said to be usable by men with diabetes or heart problems and those who have undergone a prostatectomy.[13]
Misoprostol
[edit]Misoprostol has a variety of uses in obstetrics and gynecology. It is also used to relieve pain from duodenal ulcers when other treatments have been ineffective.[14]
Misoprostol can be used to induce labor in patients at the end of pregnancy. It causes cervical ripening, or the thinning and shortening of the cervix in preparation for birth. It also causes uterine contractions, allowing the body to expel the baby. For this purpose, it is placed inside the vagina.[8][15]
Misoprostol is also used to prevent and treat postpartum hemorrhage (PPH), or uncontrolled bleeding following childbirth. The World Health Organization estimates that PPH causes 70,000 maternal deaths each year.[16] Misoprostol induces uterine contractions, encouraging the uterus to shrink after childbirth. This shrinking puts pressure on blood vessels on the uterus, forcing them to close rather than continue to bleed. To prevent PPH, misoprostol is given orally or dissolved under the tongue immediately after delivery. To treat PPH, it is given orally, dissolved under the tongue, or placed in the rectum.[17]
For the management of miscarriage in the first trimester of pregnancy, misoprostol is used to completely empty the uterus. This is important because the patient may develop an infection if they retain the products of conception. Misoprostol causes uterine contractions, forcing the body to expel the pregnancy. It can be used alone, but it is more effective in conjunction with mifepristone. Misoprostol is swallowed, placed inside the vagina, or dissolved in the mouth for this purpose.[18]
Misoprostol can also be used to induce abortion. It can be used on its own or following administration of mifepristone. Misoprostol may be used up to and after 28 weeks gestational age to terminate a pregnancy;[17] however, it is typically only used in the first trimester. Later abortions are less common and are usually performed surgically, at least in the United States.[19] For first-trimester abortion, it is dissolved in the cheek or under the tongue; for later abortions, it can also be given as a vaginal suppository. Misoprostol may also be used to ripen the cervix in preparation for surgical abortion.[20]
Misoprostol is given orally for the treatment of ulcers. It interacts with prostaglandin receptors in the stomach to reduce secretion of stomach acids. It increases the production of gastric mucus, which forms a protective coating against stomach acid. Finally, misoprostol encourages the production of bicarbonate, which is a base that counteracts stomach acid.[7] The medication can also protect against stomach ulcers in people who take NSAIDs such as ibuprofen on a daily basis.[21]
Misoprostol can cause nausea, nausea, stomach pain, and stomach cramps.[22] In rare cases, it may lead to uterine rupture, which requires immediate emergency surgery.[17]
Other uses
[edit]Prostanoids, including alprostadil, do not reduce the risk of limb amputation but may offer a slight improvement in rest-pain and leg ulcer healing in persons with critical limb ischemia.[23]
Preventative administration of alprostadil may reduce the risk of kidney injury (specifically contrast-induced nephropathy) in persons having cardiac angiography or percutaneous coronary intervention.[24][25]
Lubiprostone is a PGE1 derivative used to treat chronic constipation. It is taken orally.[9] Common side effects include diarrhea, vomiting, and abdominal pain.[26]
References
[edit]- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e "Alprostadil". The American Society of Health-System Pharmacists. Archived from the original on 16 January 2017. Retrieved 8 January 2017.
- ^ a b c Hew MR, Gerriets V (3 April 2023). "Prostaglandin E1". StatPearls. PMID 31536236 – via National Library of Medicine.
- ^ a b c Northern Neonatal Network (208). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (5 ed.). John Wiley & Sons. p. 2010. ISBN 9780470750353. Archived from the original on 13 January 2017.
- ^ British National Formulary (BNF) (69th ed.). British Medical Association. 2015. p. 569. ISBN 9780857111562.
- ^ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 185. ISBN 9780470015520. Archived from the original on 13 January 2017.
- ^ a b c Dudzinski DM, Serhan CN (2017). "Pharmacology of Eicosanoids". In Golan DE, Armstrong EJ, Armstrong AW (eds.). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (4th ed.). Wolters Kluwer. ISBN 9781451191004.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b Dietrich E, Rubin D (2023). "Anti-Inflammatory, Antipyretic, and Analgesic Agents". In Whalen K, Lerchenfeldt S, Giordano C (eds.). Pharmacology (8th ed.). Wolters Kluwer. pp. 613–633. ISBN 9781975170554.
- ^ "Lubiprostone: Drug Information". UpToDate Lexidrug. Wolters Kluwer. 2025.
- ^ Meller SM, Stilp E, Walker CN, Mena-Hurtado C (June 2013). "The link between vasculogenic erectile dysfunction, coronary artery disease, and peripheral artery disease: role of metabolic factors and endovascular therapy". The Journal of Invasive Cardiology. 25 (6): 313–319. PMID 23735361.
- ^ Vogel Anderson K, Atkinson K (2023). "Drugs for Urologic Disorders". In Whalen K, Lerchenfeldt S, Giordano C (eds.). Pharmacology (8th ed.). Wolters Kluwer. pp. 670–679. ISBN 9781975170554.
- ^ "Vitaros- New Erectile Dysfunction Topical Treatment". Meds4All.co.uk. Archived from the original on 11 February 2015.
- ^ Krugh M, Patel P, Maani CV (11 December 2024). "Misoprostol". StatPearls. PMID 30969695 – via National Library of Medicine.
- ^ Goldberg AB, Greenberg MB, Darney PD (January 2001). "Misoprostol and pregnancy". The New England Journal of Medicine. 344 (1): 38–47. doi:10.1056/NEJM200101043440107. PMID 11136959.
- ^ "WHO postpartum haemorrhage (PPH) summit". World Health Organization. 29 September 2022.
- ^ a b c "Misoprostol: Drug information". UpToDate Lexidrug. Wolters Kluwer. 2025.
- ^ Hamel CC, Snijders MP, Coppus SF, Vandenbussche FP, Braat DD, Adang EM (2022). "Economic evaluation of a randomized controlled trial comparing mifepristone and misoprostol with misoprostol alone in the treatment of early pregnancy loss". PLOS ONE. 17 (2): e0262894. Bibcode:2022PLoSO..1762894H. doi:10.1371/journal.pone.0262894. PMC 8827447. PMID 35139105.
- ^ Diamant J, Mohamed B, Leppert R (25 March 2024). "What the data says about abortion in the U.S." Pew Research Center.
- ^ Morris JL, Winikoff B, Dabash R, Weeks A, Faundes A, Gemzell-Danielsson K, et al. (September 2017). "FIGO's updated recommendations for misoprostol used alone in gynecology and obstetrics". International Journal of Gynaecology and Obstetrics. 138 (3): 363–366. doi:10.1002/ijgo.12181. hdl:2027.42/138200. PMID 28643396.
- ^ Watkinson G, Hopkins A, Akbar FA (1988). "The therapeutic efficacy of misoprostol in peptic ulcer disease". Postgraduate Medical Journal. 64 (Suppl 1): 60–77. PMID 3138682.
- ^ "Misoprostol (Cytotec)". Cleveland Clinic Health Library. Cleveland Clinic.
- ^ Vietto V, Franco JV, Saenz V, Cytryn D, Chas J, Ciapponi A (January 2018). "Prostanoids for critical limb ischaemia". The Cochrane Database of Systematic Reviews. 1 (1): CD006544. doi:10.1002/14651858.CD006544.pub3. PMC 6491321. PMID 29318581.
- ^ Ye Z, Lu H, Guo W, Dai W, Li H, Yang H, et al. (November 2016). "The effect of alprostadil on preventing contrast-induced nephropathy for percutaneous coronary intervention in diabetic patients: A systematic review and meta-analysis". Medicine. 95 (46): e5306. doi:10.1097/MD.0000000000005306. PMC 5120914. PMID 27861357.
- ^ Xie J, Jiang M, Lin Y, Deng H, Li L (August 2019). "Effect of Alprostadil on the Prevention of Contrast-Induced Nephropathy: A Meta-Analysis of 36 Randomized Controlled Trials". Angiology. 70 (7): 594–612. doi:10.1177/0003319719825597. PMID 30669852. S2CID 58950588.
- ^ "Lubiprostone: Drug Information". UpToDate Lexidrug. Wolters Kluwer. 2025.
