Wikipedia talk:WikiProject Chemistry/Archive 34
| This is an archive of past discussions on Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 30 | ← | Archive 32 | Archive 33 | Archive 34 | Archive 35 | Archive 36 | → | Archive 40 |
Occupational safety & health
Hi lovely chemists! User:Smokefoot suggested that I wander by here and let you all know what I've been up to. I'm User:Keilana, the current Wikipedian-in-Residence at NIOSH, the National Institute for Occupational Safety and Health, which is a US federal research agency. I hadn't realized when I went around telling WikiProjects about this collaboration that I'd be writing about chemistry as much as I seem to be - so here I am! I'll probably be mostly working on safety information on various chemicals, especially since NIOSH puts out the Pocket Guide to Chemical Hazards. If there's anything I can do to help you get information about a specific compound, or connect you with researchers and resources, please let me know. And of course, if you have any questions or concerns about my work, my talk page is always open. Cheers! Emily Temple-Wood (NIOSH) (talk) 19:23, 22 January 2015 (UTC)
- Emily, welcome and keep asking, although responsiveness at this talk page can be variable. Here is one over-arching comment or even caution, and these comments only reflect my views, not necessarily consensus. The articles on chemicals are intended to be useful to chemists and those interested in learning or using chemistry. All editors know that we could completely overwhelm each of these articles with safety information regarding proper handling and a laundry list of all the consequences of contact with chemical substances (invariably involving some dizziness, nausea, skin irritations, etc). We choose not to summarize all of the safety-tox-consequences. Instead we defer to the MSDS, which we carefully (hopefully) link in every chemical article. This approach is summarized in our style guide Wikipedia:Manual of Style/Chemistry#Safety, which states "The [safety] information in the Chembox is sufficient for most compounds." The exceptions to this approach are chemical species that are acutely toxic (cyanide, etc). Articles on compounds that are in heavy use but are not always acutely toxic, typically also have sections on safety, but usually not long ones. --Smokefoot (talk) 02:48, 23 January 2015 (UTC)
- Thanks for the response! I'm obviously coming from a different perspective, but I'm sure we can find a balance. I think that chemicals in heavy industrial use could do with some explanation of their dangers in the workplace, whereas chemicals that you're only going to find in a specialized chem lab are less important. This is definitely a conversation that we can continue to have! Emily Temple-Wood (NIOSH) (talk) 02:56, 23 January 2015 (UTC)
- I agree with Emily (and would extend her comment to cover household chemicals). I also think articles on chemicals - and other chemistry topics - are also supposed to be of interest - and useful - to the general readership. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 09:16, 23 January 2015 (UTC)
- I agree with Smokefoot. I read WP articles about chemicals because I want to learn about the chemicals. I don't want the articles cluttered up with a lot of safety information. But I believe that every chemical article should have an easily-found link to safety sheet, preferably in the info box so that potential users of the chemical will know where to look. Maproom (talk) 10:38, 23 January 2015 (UTC)
- Hi Emily, it’s nice to see someone specifically addressing safety issues, I'm sure that it's something that people are looking for. As Smokefoot points out MSDS's are a good source of information regarding the safety of compounds, however there is a slight issue in that different countries have different regulations, so depending on where on the internet you get your MSDS the hazards may be described differently. For example, as you're U.S. based I assume that NFPA 704 features in your way of looking at things, whereas here in the E.U. we're using the GHS system (which is replacing the ‘R phrases’ and ‘S phrases’ you may be familiar with). The current Chembox is able to support all of these different systems and provides a nice way of listing everything where it won’t get in the way of the main text. If you're going to take that route I would suggest that GHS hazard statements feature in some way as that system was put out by the U.N. and is supposed to be universal. --Project Osprey (talk) 11:35, 23 January 2015 (UTC)
- A useful conversation. The following recent edit at dichloromethane seems too (to me at least) generic and completely covered by the MSDS "Symptoms of acute overexposure to dichloromethane via inhalation include: difficulty concentrating, dizziness, fatigue, nausea, headaches, numbness, weakness, and irritation of the upper respiratory tract and eyes. More severe consequences can include suffocation, loss of consciousness, coma, and death." I do not think that Wikipedia needs to tell people that breathing volatile chemicals (aside from noble gases, SF6 and a rare few others ) makes one sick or to detail the nature of the sicknes. Thanks, --Smokefoot (talk) 13:02, 23 January 2015 (UTC)
- Smokefoot makes a good point here. Wikipedia does not need to point out the typical hazards associated with exposure to nearly any chemical. This really falls outside the scope of an encyclopedia article. The safety section should focus on potential hazards that are significant, notable, uncommon, unexpected, etc. For the routine safety information we can (and should) provide external links to authoritative resources such as MSDS sheets, occupational health and safety websites, etc. -- Ed (Edgar181) 13:21, 23 January 2015 (UTC)
- I'm not clear on that. Breathing in inert gasses like Helium or Nitrogen won't keep you alive, but as long as you also get enough oxygen it won't hurt you either. The symptoms Smokefoot describes seem like they are caused by actual exposure to the chemical, not by just "breathing stuff other than air". It seems similar to the difference between non-toxic, non-caloric substances like aspartame and sucralose (which are not nourishing but also not damaging) and toxic non-caloric foods (which are not nourishing and will hurt you if you eat them). 0x0077BE (talk · contrib) 13:34, 23 January 2015 (UTC) 0x0077BE: I rephrased my remark, thanks for pointing out the noble gas situation.--Smokefoot (talk) 13:41, 23 January 2015 (UTC)
- A little information on the pharmacology might not be a bad idea for common (or interesting) compounds, just so long as it follows WP:MEDRS. Regarding DCM I've moved the information of the PEL to the chembox, as there's an option for it. Very few chemboxes contain information of exposure limits - part of this might be because we don't really have the right fields. Currently we only have the options to enter PEL (U.S. centric) and TLV (again U.S. centric and its a safety rather than regulatory value). As we now have a direct line to NIOSH perhaps we could review those options and see if we need to add any new fields? --Project Osprey (talk) 13:37, 23 January 2015 (UTC)
- Excessively detailed health and safety information could potentially violate the medical advice prohibition, so handle with care. Roger (Dodger67) (talk) 13:45, 23 January 2015 (UTC)
- Thanks for all the input! I really appreciate it. My one concern with linking to the MSDS is that the lay reader may not understand the MSDS. I remember being a beginning chemistry student and having no idea how to read through an MSDS to get what was useful, so I think including basic information could be a good addition in cases where a chemical is widely used industrially. I know that when I'm writing medical articles, I have a lot of trouble remembering that I am writing for the proverbial "smart 15-year-old" and not just doctors and nurses, so things that may seem obvious to me (epinephrine raises your heart rate!) may not be obvious to the lay reader. [[User:Project Osprey}Project Osprey]], I'm happy to go through and add PELs and TLVs to infoboxes as a start. We can also talk to researchers and see what they think we could add - NIOSH also has contacts at other regulatory agencies and we could use those to sort out what other fields could be useful. Again, my hands are tied a bit on the US-centric issue since NIOSH is a US agency and I'm a US-based person, but I think we can sort some of that out. Best, Emily Temple-Wood (NIOSH) (talk) 14:46, 23 January 2015 (UTC)
- Forgot to add - I can add PELs but NIOSH doesn't do TLVs, just Recommended Exposure Limits (which would be a very useful thing to add to the chembox) and the short-term exposure limit, which is another useful thing to add. What do you all think about those as parameters? Best, Emily Temple-Wood (NIOSH) (talk) 14:55, 23 January 2015 (UTC)
- Well, to start with any data is better than no data. I think the idea with MSDS's was to cite them as references and then transcribe the hazard codes into the relevant Chembox section. I'm aware that those codes are a rather 'dry' description of the hazards but they are all defined against the same standards, so when is says 'toxic' rather than 'harmful' that really means something. Regarding the regulatory stuff, I'm not familiar with the current U.S. standard - so it would be good to check that TLV's and PEL's are still the standard values used before starting on that.
TLV's are the limit at which things get dangerous, so they're a medical estimate and not a regulatory limit, as such they should be fairly universal (and a lot more useful than LD50)nevermind! If you want to have new fields added to the chembox then you'd be best to raise it at Wikipedia_talk:Chemical_infobox. The other question of course is where do you being? The popular pages list might show you some low hanging fruit. Do you have any particular areas of interest (or assigned goals?) and is there any particular support that you'd like from us? --Project Osprey (talk) 15:24, 23 January 2015 (UTC)
- @Project Osprey: Well, I have quite a lot of data! :) TLVs are the toxicology society and RELs/PELs are from NIOSH and OSHA, respectively. So we're good on that! I'll go put something on the chembox talk page to see if people would find the short-term exposure limits helpful as well. I just spoke to some of my NIOSH colleagues and they did say that they will be reaching out to their contacts and collaborators at other OSH agencies around the world to get their guidelines and have them share what would be useful. So watch this space - more international regulatory data (and other data!) will be coming your way! My personal area of interest is carcinogens, but I have pretty free rein on what to work on. So if there's anything that the project is specifically focusing on, or if any editor needs resources, I'm happy to help out. I of course want feedback from everyone and if I contravene guidelines or screw up in some other way, please do let me know. The most helpful thing in the world would be you letting me know what you need! And, thank you so much for your dialogue and comments, it's good to get to know you all. All the best, Emily Temple-Wood (NIOSH) (talk) 16:11, 23 January 2015 (UTC)
- Best wishes to you and your colleagues but please dont become too evangelical in your campaign. If you and your colleagues develop a huge amount of content, we can create a new article on Toxicology of DCM or Toxicology of Chlorinated Solvents. We have that pattern of establishing stand-alone safety articles for a variety of important materials - Cyanide poisoning, fluoride toxicity, CO poisoning. But we need to do better than "Symptoms ... via inhalation include: difficulty concentrating, dizziness, ... coma, and death." --Smokefoot (talk) 18:16, 23 January 2015 (UTC)
- Well, to start with any data is better than no data. I think the idea with MSDS's was to cite them as references and then transcribe the hazard codes into the relevant Chembox section. I'm aware that those codes are a rather 'dry' description of the hazards but they are all defined against the same standards, so when is says 'toxic' rather than 'harmful' that really means something. Regarding the regulatory stuff, I'm not familiar with the current U.S. standard - so it would be good to check that TLV's and PEL's are still the standard values used before starting on that.
- Smokefoot makes a good point here. Wikipedia does not need to point out the typical hazards associated with exposure to nearly any chemical. This really falls outside the scope of an encyclopedia article. The safety section should focus on potential hazards that are significant, notable, uncommon, unexpected, etc. For the routine safety information we can (and should) provide external links to authoritative resources such as MSDS sheets, occupational health and safety websites, etc. -- Ed (Edgar181) 13:21, 23 January 2015 (UTC)
- Thanks for the response! I'm obviously coming from a different perspective, but I'm sure we can find a balance. I think that chemicals in heavy industrial use could do with some explanation of their dangers in the workplace, whereas chemicals that you're only going to find in a specialized chem lab are less important. This is definitely a conversation that we can continue to have! Emily Temple-Wood (NIOSH) (talk) 02:56, 23 January 2015 (UTC)
I agree with Smokefoot and others on this - chemical articles don't need bogging down with a great list of health risks. I do not think that extracting useful information from an MSDS is particularly difficult at all - isn't that the point? With that said, I really respect that a gov organisation is funding adding information to wikipedia and I appreciate that Emily is not the one setting her own job spec. I hope that we can find a pragmatic compromise on our views. Testem (talk) 20:08, 23 January 2015 (UTC)
Comment: I am opposing such unreferenced additions. --Leyo 11:47, 24 January 2015 (UTC)
- Easily fixed, providing we agree to accept web-citation from NIOSH. --Project Osprey (talk) 12:07, 24 January 2015 (UTC)
- @Leyo: @Project Osprey: My apologies, I didn't know you had to cite stuff in the chembox. Everything I'm adding there comes from the NIOSH Pocket Guide, which seems to be linked in most chem articles. I read through the chembox documentation and the MOSCHEM and it wasn't mentioned, so perhaps that would be something helpful for chem newbies. :) How would you like me to cite the PEL in the chembox? With a normal inline citation or is there some other way you prefer? Best, Emily Temple-Wood (NIOSH) (talk) 00:19, 25 January 2015 (UTC)
- There is no PEL_Ref parameter. Should we add this to the Template:Chembox Hazards? If not then inline reference is the way to go. Perhaps we need a template to assist with NIOSH references if there are going to be a lot of links to the same data source. Graeme Bartlett (talk) 00:39, 25 January 2015 (UTC)
- +1 --Leyo 00:44, 25 January 2015 (UTC)
- I now see there is a Template:PGCH already. Graeme Bartlett (talk) 23:34, 25 January 2015 (UTC)
- There is no PEL_Ref parameter. Should we add this to the Template:Chembox Hazards? If not then inline reference is the way to go. Perhaps we need a template to assist with NIOSH references if there are going to be a lot of links to the same data source. Graeme Bartlett (talk) 00:39, 25 January 2015 (UTC)
- @Leyo: @Project Osprey: My apologies, I didn't know you had to cite stuff in the chembox. Everything I'm adding there comes from the NIOSH Pocket Guide, which seems to be linked in most chem articles. I read through the chembox documentation and the MOSCHEM and it wasn't mentioned, so perhaps that would be something helpful for chem newbies. :) How would you like me to cite the PEL in the chembox? With a normal inline citation or is there some other way you prefer? Best, Emily Temple-Wood (NIOSH) (talk) 00:19, 25 January 2015 (UTC)
The usage of Buckyball is under discussion, see talk:buckyball -- 65.94.40.137 (talk) 09:37, 27 January 2015 (UTC)
(hydroxyethyl)methacrylate
what (hydroxyethyl)methacrylate? 76.120.164.90 (talk) 01:41, 26 January 2015 (UTC)
- What are you trying to ask about (Hydroxyethyl)methacrylate? Graeme Bartlett (talk) 03:36, 26 January 2015 (UTC)
- it is an anion, what's the cation for the data? — Preceding unsigned comment added by 76.120.164.90 (talk) 04:07, 27 January 2015 (UTC)
- No it is a neutral molecule. pKa/pKb is 13.82, so in very alkaline conditions it could lose a proton, or gain a hydroxyl group to make a negative ion. Graeme Bartlett (talk) 11:37, 27 January 2015 (UTC)
- The "cation" you're referring to is hydroxyethyl. And the "anion" is methacrylate. That is to say, it is the hydroxyethyl ester of methacrylic acid. But like Bartlett says, it is still a neutral molecule. Hope that clears things up. Jynto (talk) 11:58, 27 January 2015 (UTC)
- it is an anion, what's the cation for the data? — Preceding unsigned comment added by 76.120.164.90 (talk) 04:07, 27 January 2015 (UTC)
Third opinion on the value 3D models
@Jynto:
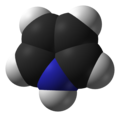
What purpose does a 3D model serve if it is not space filling? To my mind it is just a duplication of the 2D model in this case, but may obscure some of the bonds if it is not rotating. With a space=filling model at least there use in seeing a clear 3D structure and being able to make a comparison of, for example, possible binding positions. If I am entirely honest I see little use for 3D models at all without the ability to manipulate them, apart from for the aesthetic appeasement of laymen. I am specifically asking because of some of the edits made by Lazord00d (talk) who "justifies" all of his edits with arguments I find difficult to understand as a chemist but apparently make sense to him as someone without "Chemical training". Testem (talk) 18:58, 9 January 2015 (UTC)
- Thianthrene is a good example where a ball-and-stick model provides additional information compared to the skeletal formula. --Leyo 20:43, 9 January 2015 (UTC)
- Good example. Is that only because it is rigid? Could the same job be done with more functionality by a space filling model? Testem (talk) 20:57, 9 January 2015 (UTC)
- In this case a ball-and-stick model is more useful than a space-filling model, especially since the non-planarity can be recognized more easily. --Leyo 17:55, 10 January 2015 (UTC)
- Good example. Is that only because it is rigid? Could the same job be done with more functionality by a space filling model? Testem (talk) 20:57, 9 January 2015 (UTC)
- Sorry Jynto, As a chemist, I'm extremely surprised by your position on this. I've justified mine with solid logic. If you feel I haven't please feel free to point out where and I'll gladly explain further. To quote myself from a recent post on Testem's talk page: "The blended type of 3d model translates directly from the formulaic model with minimal differences, except that they show atoms that are omitted in the 2d models, therefore they help the broadest range of minds grasp the concept." I do not feel that this logic is flawed.
- Laterz Lazord00d (talk) 22:04, 9 January 2015 (UTC)
- @Lazord00d: Which omitted atoms do they include? Why is this helpful to a non-chemist? Testem (talk) 11:39, 11 January 2015 (UTC)
@Testem:: 2d diagrams omit most Hydrogens and their bonds to Carbons. I know it is assumed by people in the know, but not by people who are new to this. Lazord00d (talk) 17:43, 29 January 2015 (UTC)
- My 2 cents, I think that the new images are often not very useful and should be employed selectively. --Smokefoot (talk) 23:30, 9 January 2015 (UTC)
- Selectively by what criteria? Point out a standard that makes sense and I'd be willing to adhere to it. Right now in terms of molecular visualization on Wikipedia it's a mish-mash of styles, there is no standard other than butthurt when someone changes something. I've had changes made to my edits before with very reasonable and logical explanations and had no problem with it. The inability of the other players here to provide a point at which I have deviated from what I consider to be sound logic indicates (at least to me) that the drive behind all this has nothing to do with fact or science.. I have explained my position numerous times and asked for explanations of theirs but all I've seen has been almost entirely opinion-based. I've asked for someone, anyone, to explain why or how editing in a manner that, due to the simplicity of the comparison, will allow for anyone to understand is a problem or flawed logic. Thus far I haven't seen any solid response to that. Lazord00d (talk) 23:43, 9 January 2015 (UTC)
- IMO selectively in this case would mean a 3D model would only be used where it makes a clear improvement to the understandability of the article, such as with Thianthrene. Testem (talk) 11:39, 11 January 2015 (UTC)
- Agreed that 3D images are of limited value, especially of arbitrarily chosen conformations of flexible molecules. The advantage of stick diagrams over space filling is that it is easier to see the underlying connectivity. Space filling may be useful, especially if the surface is color coded by some property like electrostatic potential. Which depiction is better depends on what you are trying to illustrate. Boghog (talk) 23:45, 9 January 2015 (UTC)
@Lazord00d: Are you sure of who you're talking to? Because you seem to think Testem's comment was written by me. @Testem: To answer your question, I think there is a value in ball-and-stick models. They show geometry, and a possible conformation, whereas the skeletal formulas don't. They are also show the scale of the molecule, if not quite as well as their space-filling counterparts. Most importantly the colour-coded spheres are much more intuitive to the non-chemist than zigzag lines will ever be, which I think is the point Lazord00d was trying to get across.
I do think there should be a place for space-filling models too. But it's difficult to draw a line in the sand about when and when not to use them. It's hard to justify a space-filling model when they don't show the bonding, and parts of the molecule are hidden behind other atoms. Making them spin is not the answer, as hard-line academic types like Leprof 2727 are dead set against them, and I must agree. Spinning models are distracting.
So I must turn the question round and ask you, Testem, what advantages do you think space-filling models serve over 'blended' ball-and-stick ones? When should one be used over the other? I'm thinking space-filling for articles with the drugbox template, and ball-and-stick for the regular chemboxes, but I can already see some places where that would not work. And when is it a good idea to use both? Jynto (talk) 23:55, 9 January 2015 (UTC)
- To clarify, I question the utility of any 3D models in a majority of articles. An arbitrary display of a single conformation of many is not useful IMO, which is why skeletal formulae are the standard.
- Colour coding may appear intuitive at first but unless there is a legend it is not useful to a non-chemist and chemists do not need the additional intuitiveness.
- If 3D models must be used then I feel space-filling varieties are of more use as they have some value in at-a-glance comparisons of molecules for receptor binding, but as I say I do not think 3D models are valuable for conformationally restricted compounds.Testem (talk) 11:39, 11 January 2015 (UTC)
- Apologies I had some trouble figuring out who was saying what. I'm not always good at stating things in the simplest manner, so sorry if I didn't word things right but Jynto is correct the features he mentioned are why I feel that the blended or stick and ball type models are best for helping even people with no chemistry experience whatsoever understand what they're looking at/reading about. Lazord00d (talk) 01:06, 10 January 2015 (UTC)
- @Lazord00d: Why do they help non-chemists understand what they are looking at? Genuine question as it has been a long time since I looked at something from this viewpoint and there may well be a use I am not seeing.Testem (talk) 11:39, 11 January 2015 (UTC)
- If I was to answer by a (perhaps quite flawed) analogy, I'd say it's that the difference between skeletal formulas and 3D models is like looking at the blueprints of a car versus seeing a photo of the actual car. Jynto (talk) 01:34, 12 January 2015 (UTC)
- Hoping I've interpreted your analogy as you intended: is that beneficial if the person puts no value in the aesthetic qualities of the car and the photo does not visualise anything better than the blueprint? Forgive my constant questions, I am trying to understand my own viewpoint better too. Testem (talk) 09:31, 12 January 2015 (UTC)
- I can't think of a better way to say it, but simply put images should look like the thing they're about. Quoted from the Manual of Style:
- "Images are primarily meant to inform readers by providing visual information. Consequently, images should look like what they are meant to illustrate, even if they are not provably authentic images." --Jynto (talk) 03:22, 14 January 2015 (UTC)
- So then surely we should be using real-colour SEM images? Ball and stick models look nothing like the molecules they represent. You didn't answer my question by linking that :( Testem (talk) 09:08, 14 January 2015 (UTC)
- Hoping I've interpreted your analogy as you intended: is that beneficial if the person puts no value in the aesthetic qualities of the car and the photo does not visualise anything better than the blueprint? Forgive my constant questions, I am trying to understand my own viewpoint better too. Testem (talk) 09:31, 12 January 2015 (UTC)
- If I was to answer by a (perhaps quite flawed) analogy, I'd say it's that the difference between skeletal formulas and 3D models is like looking at the blueprints of a car versus seeing a photo of the actual car. Jynto (talk) 01:34, 12 January 2015 (UTC)
- @Lazord00d: Why do they help non-chemists understand what they are looking at? Genuine question as it has been a long time since I looked at something from this viewpoint and there may well be a use I am not seeing.Testem (talk) 11:39, 11 January 2015 (UTC)
From my perspective the biggest benefits over 2d are the visible presence of all atoms normally omitted in 2d formulaic models, and visualization of angles which, while there is a system of markings in the 2d system to describe them, are much more easily understood visually whenever possible. The more complex the 3d model, the more angles involved, the harder it gets to render in a static 3d image without masking atoms and sometimes whole structures. This applies to both space-filling and ball-and stick models but it's much worse with the former. I've seen rotating .gif models here of both types and dislike them quite a lot, they're distracting and often the axes positions and visual perspective are not optimal. Also, given that Wikipedia is a publicly edited encyclopedia viewed by millions (billions even?), the people who will ultimately view these images in the context of their articles will span an enormous range in every conceivable way. For the majority of them, the most direct comparison between 2d and 3d is what is most helpful for understanding what they are seeing just like the vast majority of people would rather look at the car than it's blueprint. Because this is Chemistry and not cars, the best solution is a blended concept, the stick and ball model. I make mine the way I do because they're most similar to the 2d models and the double-bonds etc correspond directly to the 2d images.
Lazord00d (talk) 16:13, 12 January 2015 (UTC)
I have to say I completely disagree with the idea that ball-and-stick and space-filling models are not useful. The three dimensional structures of molecules are one of their most important characteristics, responsible for many of their physical, chemical and biological properties. Our readers are not simply either chemists or non-chemists, they range from the general public, through school and university students to professional scientists. In general, I find ball-and-stick models more useful as it is clearer which atoms are where - the structure is more open. Their main use is to help readers to visualise the molecules and highlight key structural features such as bond lengths and angles that are not indicated in any great detail in the 2D formulae. Space-filling models give a useful indication of the relative volumes of different atoms, and how closely two or more molecules can pack together.
In a typical Wikipedia article on a molecular organic compound, the skeletal formula is how the molecule is typically depicted in textbooks and journal articles. The drawing conventions can be obscure to the untrained eye, so I began adding 3D models for a wide range of important compounds around 2006. There were a few such images before I began, and now thanks to the efforts of a dozen or so editors there are thousands.
Regarding flexible molecules, yes there are plenty of them, but many important molecules are actually quite rigid - most drugs have a rather inflexible core at least. There is some harm done in conveying the impression that all molecules are static, which they are not, but having the image is probably better on balance than not having one, especially if the flexibility is described in the text.
If you want an idea of how useful and important molecular models are, open a standard undergraduate organic or inorganic chemistry textbook, such as Housecroft's Inorganic Chemistry, and you will see that half the ink is used on them! --Ben (talk) 19:15, 11 January 2015 (UTC)
- p.s. In my images, the colour scheme is generally given if you click on the image and go to its description page. You can also work out which element is which colour by comparing the structural formula and the 3D model. --Ben (talk) 19:16, 11 January 2015 (UTC)
For a layman a ball-and-stick model also seems very rigid. Imho 3d models also distort the reality, because atoms are not colorful. When a layman wants to reach higher, he must be able to derive knowledge from the plain old structural formula. Who does really need to know the 3-dimensional structure if he's not a researcher?--Kopiersperre (talk) 11:56, 13 January 2015 (UTC)
I think the answer to that would be 'almost everyone who learns visually'. Some people, possibly including yourself, might just find it very easy to memorise the structures of lots of different compounds, but to almost everyone else, a skeletal formula is a collection of black lines on a white background, and they all look the same. Molecular models are visually distinctive in a way that skeletal formulas are not, which is why readers with visual learning skills can learn the structure of a compound by remembering what it looks like. This also it makes it a lot easier to appreciate the (otherwise quite subtle) differences between compounds, because again molecular models tend to be visualy distinctive from each other. Flat geometry looks very different from aliphatic geometry in a molecular model. An amide group looks very different from a sulfonamide group, and so on. And many readers will associate what they learn about a particular compound with their visual memory of the molecular model they saw on that page.
I'm hoping this doesn't seem entirely foreign to you, because a lot of us are visual learners. Even if you don't learn anything from the model, it is still a tool to assist your learning. It almost doesn't matter whether you're a layman or an expert. The same learning techniques apply. --Jynto (talk) 03:22, 14 January 2015 (UTC)
I just thought I'd use some images to illustrate my point. Based on what you can see above, you'd never get sulfamide confused with urea if you just remember that it contains the yellow sulfur atom, and urea doesn't. Similarly you wouldn't get 1-pyrroline mixed up with pyrrole if you can remember from the molecular models that it isn't fully planar, whereas pyrrole is. --Jynto (talk) 04:03, 14 January 2015 (UTC)
- @Jynto: I do completely understand where you are coming from, and I completely agree that the use of 3D models of any variety is very useful, possibly even essential, in the examples you give. Do you agree though that where the structure is not constrained, the 3D models are much less useful, especially where the conformation conceals one or more parts of the molecule? Testem (talk) 09:03, 14 January 2015 (UTC)
- @Jynto: A chemist should think sulf-ur when he hears sulf-amide.
- Maybe I'm contaminated by german conservativism. In school we were allowed to play with molecular model sets. In university books you will find some, but not many 3d models.--Kopiersperre (talk) 12:08, 14 January 2015 (UTC)
I don't think anything relevant to this discussion has to do with your country or which way you lean in life ;-). The atoms are all the same.. even German ones. A well-rounded encyclopedia should appeal to users from "school" to the university and beyond. That's why, when I upload a model, it's of the 3d stick-and-ball type that I position as closely to the existing 2d model as possible.
Lazord00d (talk) 17:30, 15 January 2015 (UTC)
Just to let you folks know: I nominated a bunch of inappropriate ball-and-stick models for deletion. --Leyo 01:04, 17 January 2015 (UTC)
Even though I'm probably talking to a wall here, I'm curious how it was determined beyond all doubt that my images are "inappropriate"? The deletion thread Leyo links to was closed mid-discussion, ending with the deletion of donated time and effort without anything to back such an extreme change (the images were found simply by searching for everything donated by "Lazord00d").
Despite the presence of a handful of opinions and one admin called Leyo with an itchy trigger finger and a personal agenda, I haven't seen anything scientifically solid to back the act of declaring everything I've worked on and donated as "inappropriate" and deleting it.
- I haven't even seen evidence you understand the objections, let alone a source for your additions. You have ignored my questions above. Testem (talk) 13:55, 28 January 2015 (UTC)
- {ping|Testem} I believe I have addressed all of your concerns with responses here in this section. I understand the criticism, but I don't think any of it outweighs the value of ease of comprehension in a public resource. Lazord00d (talk) 19:19, 29 January 2015 (UTC)
In fact that's what continues to bring me back here even though I'm past soured on this place, I'm hoping that as many people as possible see how it works here on Wikipedia. They need to understand that truly unbiased science is not and will not be fostered, encouraged or even considered worthy of a cited rebuttal as long as there are "Leyos" and their followers running the game. People who are holding anyone who has some color to their character and a sense of humor responsible for that reprehensible crime by stalking them and holding everything they do up to "Leyo" standard, and if not (regardless of science or citation) you get the "treatment". Example here (at end of section): https://wiki.riteme.site/wiki/Talk:Etizolam#Molecular_structure. Granted my tone isn't always friendly, but the act of forcing a person to comply with a concept that isn't a rule or part of the official style outline amounts to strongarming. I quote Leyo and myself here:
[Quote] "Try to follow User:Benjah-bmm27/MakingMolecules and you will be surprised on how quickly the “noise” will decline. ;-) --Leyo 08:24, 22 January 2015 (UTC)
Yeah I'll bet. How does that not amount to hounding me to comply with rules that aren't necessarily the only correct way to create models? How about this: I kinda like the idea of not ever contributing any useful content to Wikipedia again. That option works best for me thanks.. sad too. I started a business years ago related to my username, and now we are one of the largest in our field which is very small and exclusive to begin with. I definitely have knowledge to share across a broad range of topics but Wikipedia truly seems more about who likes which person's personal characteristics than whether factual data is present. I have zero interest in complying with people just to make them feel "in control". If I was easily pushed around do you think the company as a whole would have been successful? lol.. I appreciate your reply Leyo but still it falls short. :-/ Lazord00d (talk) 15:15, 22 January 2015 (UTC)" [/quote]
Basing admin actions on personal opinion without a thing backing them up guarantees that bias will always remain and will be enforced as a rule. The fewer people waste their valuable time trying to help like I did the better. It's a PSA! And the bold is because this is a long section and it's easy to get lost.. it would be good to use a separator of some sort between comments imo. See? That's an opinion that isn't masquerading as scientific fact! In-conceivable!!!! :-)Lazord00d (talk) 23:32, 27 January 2015 (UTC)
Well darn it. I'd be deeply sorry to hear that Wikipedia has lost a contributor because of this. Especially since it was me who started this whole war in the first place with my edit to MDMA. While we're being truly honest here, here's another opinion that isn't disguised as scientific fact. Your molecular models were, for lack of a better word... ugly. They were low res and anti-aliased. They looked kinda like the automated molecular diagrams you'd see PubChem or somesuch. And yes, the alternating double bond thing is ugly. I won't say it's any better or worse than the dotted line for showing the molecule's bonding, but you didn't make yourself very popular when you wrote edit summaries to the effect that yours were the 'improved' versions.
To add to that, like 99% of the images here already used the dotted line representation. I know you do appreciate consistency, being an advocate for it yourself, so perhaps you can understand why some Wikipedians would like your images to be consistent with what's already here. Again, I can't speak for everyone, but we get quite attached to everything that's here - this wonderful encyclopaedia of everything we've built for ourself. I know you yourself have felt something similar, because you used emotionally charged words like "donated" when you describe your time spent here. There's nothing wrong with feeling that way.
And yes, we like Ben Mills' images, guilty as charged. They are both scientifically informative and beautiful, and of course consistent. When I first saw that only some chemistry articles had them, I wanted to see more of them. I actually got my start in molecule-making by trying to copy Ben, hence some of my embarrassing early attempts to photoshop his images into something else. I did eventually develop my own way of doing them, but I never strayed too far from the style that first inspired me.
I suppose we feared you might replace every molecular image with a contribution of your own, and throw all that good stuff out with the bathwater. That would be the logical way to make things consistent with what you desired. And since you made a lot of edits in quick succession, you'll have to forgive us for overreacting.
I can't claim to speak for Leyo, or why he let these minor disagreements escalate into all this... vitriol, but I daresay you too had a part to play in this. I think you'd be happier on Wikipedia if you tried harder to make your contributions blend in with the existing content. If the double-bond thing still makes you mad, just try to think of it as something that must be done to improve Wikipedia's consistency. Maybe give Ben's guides another try. I know they don't go massively into depth, but if you have any questions and issues with DS Visualizer, you're welcome to ask me.
Please don't get banned again. I wouldn't want you to miss POTY 2014 because of this. :-)
Sincerely
- Jynto (talk) 02:20, 28 January 2015 (UTC)
That's nice thanks for your thoughts on my work, good thing I care not or I might get offended lol. I haven't been banned yet. I would have to assume that's (hopefully) because personal attacks aren't the crux of my argument and I've provided citation of that fact several times. That's what was required of me by my educators throughout life, but somehow that's not enough for this place? It's more than the opposition has done. I apologize if I upset anyone while defending my position but there is definitely something off and very unscientific about all of this and I will defend my position until the job is done. You can apply that anywhere in my life. The fact that someone thinks another editors contributions are "ugly" is of no consequence scientifically. All of my images were deleted without scientific merit. I was "ordered" to comply with an opinion as if it were fact or face continued opposition which is not scientific. I definitely played a part I won't deny that.. but I would have been a whole different person if the opposition would have supported their case or offered constructive criticism on things like anti-ailiasing etc instead of deleting without reference. Jus' sayin'.
Lazord00d (talk) 02:32, 28 January 2015 (UTC)
Well, if you're this determined to play the villain, I shan't stop you. Just remember I gave you a chance. Either way, I'll be taking my seat. The next act of this pantomime looks set to be an entertaining one.
Jynto (talk) 02:53, 28 January 2015 (UTC)
How was that playing villian? Asking for some proof to back opposing actions is being a villain? Expecting an encyclopedia to respect the need for citations and unbiased science is being a villain? Now you're not making sense.. To quote directly from WP:Consensus:
"In determining consensus, consider the quality of the arguments, the history of how they came about, the objections of those who disagree, and existing policies and guidelines. The quality of an argument is more important than whether it represents a minority or a majority view. The arguments "I just don't like it" and "I just like it" usually carry no weight whatsoever."
Lazord00d (talk) 03:28, 28 January 2015 (UTC)
- You don't have any citations or unbiased science - in fact your arguments seem to be primarily based around you "just liking" the images you have created. Testem (talk) 14:06, 28 January 2015 (UTC)
@Testem:: Citations supporting the configuration I use for my 3d models are found embedded in each image and are as follows:
Own work via jmol and 2d diagram. Citations supporting the specific format I use for my images are:
- "Textbook-Integrated Guide to Educational Resources: Aromatic Hydrocarbons: Physical Properties and Sources". Chemical Education Digital Library.
- "5-allyl-benzo-1,3-dioxole". SpringerMaterials: The Landolt-Börnstein Database.
- Zoë J. Wood. "3D Molecule". users.csc.calpoly.edu.
Lazord00d (talk) 17:40, 29 January 2015 (UTC)
- Just two observations, totally biased: (1) editors who have a single focus - drawing for example - often have modest grasp of chemistry, which detracts from their sense of when a drawing is helpful and other aspects. (2) ugly/bad chemical drawing is something that professional chemists know when they see it, but can be difficult to specify. Determined drawers would be frustrated by this observation, understandably because they are determined draw even if their services are not sought or even helpful. Advice: don't be single themed author, do various jobs related to content and non-chemical areas to gain perspective. --Smokefoot (talk) 15:59, 28 January 2015 (UTC)
Proposal
3D images should only be used when there is a clear and easily demonstrated benefit to understanding conferred by them.
For example, in structures where the shape is planar or conformationally constrained, or where the structure represents the most active conformational isomer.
- Agree - This would ensure that 3D models are permitted where useful but otherwise edit wars with Lazord00d can be avoided. Testem (talk) 14:04, 28 January 2015 (UTC)
- Disagree - We should not have to enact a blanket ban on a certain kind of image just to stop the disruptive actions of one editor. - Jynto (talk) 17:43, 28 January 2015 (UTC)
- Agree. The four examples above show why: the 2D versions are much easier to understand, at least for the undersigned non-specialist. Maproom (talk) 18:18, 28 January 2015 (UTC)
- @Maproom: I do not think anyone is proposing doing away with the 2D structures. VQuakr (talk) 04:24, 29 January 2015 (UTC)
- Disagree. Different individuals learn in different ways. Understanding 3D conformations is critical to understanding chemistry, and understanding 3D bond angles from a 2D skeleton is going to vary from person to person. As Jynto above, we should not be throwing out most or all 3D models just because one editor is disruptively inserting incorrect ones - baby, bathwater, etc. VQuakr (talk) 04:24, 29 January 2015 (UTC)
- Agree. Seems like a no-brainer: we only want drawings when they enhance vs clutter or even possibly mislead. Determined drawers often seem to be contributing to satisfy some internal urge to deposit images in Wikipedia. Ben Mills's work is a good counter example of a chemist who knows when to use good graphics and when not to.--Smokefoot (talk) 16:04, 29 January 2015 (UTC)
- Disagree. Ease of comprehension is essential in an encyclopedia which is intended for all audiences regardless of foreknowledge. Including verifiable 3d models for every molecule would be ideal from my view, regardless of who makes the images. In the physical real world the configurations of these molecules will not necessarily look exactly like either the 2d OR 3d model. Many times things are much messier in reality. These images are intended only to help with comprehension and translation between the 2d and 3d configuration. As has already been stated, ball-and-stick models show the greatest amount of detail compared to 2d and most other 3d configurations.
Lazord00d (talk) 17:34, 29 January 2015 (UTC)
- Ambivalent. I support the principle of using 3D images intelligently, to create the best possible articles for our readers. However, deciding what is best is fraught with difficulty. Obviously, grossly incorrect, misleading or confusing images should be removed. In many cases, though, the images are at least reasonably good and the decision turns to elegance and clarity. I am concerned that having strict rules about when to include a 3D model will lead to a lot of bureaucratic procedures and edit wars. I think the best approach is for the well-established, experienced and chemically educated members of this Wikiproject to make the final decision on whether to include a 3D image in any particular article. There needs to be a case-by-case approach to this complicated question. The issue of static 3D models misrepresenting molecules as rigid when they are in fact mostly flexible is not very important, in my opinion. To understand that level of chemical sophistication, a reader needs to be familiar with the rather simpler idea of what a molecule is, i.e. a collection of atoms that are bonded together and move as a discrete entity. If the flexibility of a molecule is very important to its properties, this can be discussed in the text and even illustrated with a series models of different conformations. The main reason I started adding 3D structures to Wikipedia articles on molecules and other substances was to give each article an easily understood and yet scientifically accurate picture of the chemical structure for those who could not quickly or easily interpret the skeletal formula. There are other benefits, especially for crystal structures which cannot normally be deduced from the formula, and for molecules such as drugs, where subtle structural details determine their important properties. Ultimately, we need expertise, skill, and good judgement more than we need new regulations. --Ben (talk) 19:33, 29 January 2015 (UTC)
- Agree – (EC: Ping pong, ping pong, ... ;-) A 3D depiction without a corresponding explanation of the significance of the depiction causes more confusion than clarity. We don't need to explain in each and very chem/drug infobox that chemical structures live in three dimensions, not two. We have other articles like conformational isomerism to explain that. I would go even farther than the proposal. Not only should the 3D structure have a clear benefit, it should also be accompanied by prose in the article that demonstrates that benefit. And per WP:V this benefit must also be supported up by reliable sources. Boghog (talk) 19:40, 29 January 2015 (UTC)
Discussion of proposal
To maintain ease of reading proposal votes. Testem (talk)
@VQuakr: Is the best way to teach about 3D structures by including them in every article where they will have no other explanation or would it be better to point users towards a dedicated article where the 3D structure is important but variable? Testem (talk) 09:15, 29 January 2015 (UTC)
It sounds obvious, but when a reader comes to an article about a chemical, they are expecting to find there a concise summary of everything Wikipedia has to offer about that particular chemical, including its 3D structure. Linking to a separate article is not concise. You cannot expect people to click through to that. And putting that information in a separate article would only be punishing those who want to see a molecule's structure and don't particularly care about the flexibility of it. Whereas 'experts' in the topic will probably already know a flexible molecule when they see one, and will not be greatly helped by this.
I understand what you're getting at though. Diagrams in textbooks have always appeared to falsely imply that molecules never move from the positions they are depicted in. While in fact, there is a diverse range of vibrations and conformational freedom in almost any molecule. Avoiding this implication is a laudable goal, and not an easy one at that. Correct me if I'm wrong, but isn't this the reason why you suggested such a policy in the first place? In that case it has nothing to do with disruptive editors, so please don't drag lazord00d into this.
Anyway, there comes a time in anyone's chemical education when they have to realise that molecules are chaotic. Now I don't know about you, but I think that removing the static molecules from Wikipedia would not aid anyone in reaching this stage faster. If anything, the idea of 'static' molecules, misleading as it may be, is a useful foundation to learn the other stuff from. It's like how you can't learn about molecular orbitals until you first learn about 'electron shells'. Reaction mechanisms are similarly chaotic. And it seems you can't learn anything in chemistry without it being, to some extent, a simplification of the truth.
That is not to say we should avoid the topic of conformational freedom (sadly a redlink at the time of writing) entirely. Articles like staggered conformation should be expanded significantly. And if the flexibility of a molecule is mentioned in the text, then that is where there should be a link to one of these articles. I have no problem with using static images at the same time to depict flexible molecules. It's the best simplification that we have.
- Jynto (talk) 14:01, 29 January 2015 (UTC)
- That is a fair point, and well thought out. I understand that users need a one stop resource, but what I'm saying is that nobody is looking for a 3D image of the molecule in 99% of cases because they are not useful. Therefore retaining the images takes away from it being a concise summary. What do you think?
- Testem (talk) 14:14, 29 January 2015 (UTC)
- Fair point, but couldn't the same be said about most lead images? By the same token, we have thousands of articles about towns where the lead image is some touristy photo of a local landmark, or even just the town's high street. You may wonder why we have those images, since they probably don't contribute directly to the reader's knowledge of the place. What about satellite photos on articles about hurricanes? Can the reader truly gain an understanding of the storm's destructive power just by looking at some swirly clouds without a size reference (unless Florida is nearby)? Nonetheless, I think that every one of these images is important, for reasons I have already tried to articulate, but here goes again: eye-catching images are memorable, and they make the rest of the article more memorable by association.
- When you learn facts, it helps to have an eye-catching image because it creates a convenient visual memory to attach those facts onto. Structural formulas are rarely eye-catching. That's what I was trying to say with the examples above, but I think I got carried away in my own explanation. My point still stands, in that we are very visually oriented creatures. Just look at some of the images around you, and think about how they might aid your memory of a particular item or concept, besides just being a pretty picture.
- If you need any further proof, see how my images have been used outside of Wikipedia as the primary illustration of certain compounds.
- I should mention that I had no hand in putting those images there, besides releasing them with a CC-Zero license. The gamma valerolactone image wasn't even used in the WP article, so they could only have found it by looking on Commons. These aren't the only examples either; they are just the only examples that I happened to find in my daily web browsing.
- We do have an article on conformational isomerism which includes the concept of conformational freedom. I have taken the liberty of creating a redirect from the later to the former. Boghog (talk) 14:15, 29 January 2015 (UTC)
- The problem I have with a 3D image of an arbitrary conformation of a flexible molecule is that at best, it does not tell you much and at worst, it is misleading (i.e., leaving the impression that this is the one and only "true" 3D representation of the molecule). Boghog (talk) 14:29, 29 January 2015 (UTC)
- See above. To summarise my arguments, it is vastly preferable to learn the configuration of a molecule, and later to learn that this is arbitrary, one of many possible configurations, than not to learn it at all. Simplification of the truth, etc, etc. - Jynto (talk) 14:39, 29 January 2015 (UTC)
Hi All! :-)
This is indeed healthy discussion. In what I'm sure is an unexpected occurrence, I agree with Jynto his argument is essentially the same as mine was to begin with. Simplicity and ease of comprehension are both beneficial in an encyclopedia which is expected to be a reference for anyone regardless of foreknowledge. Lazord00d (talk) 17:27, 29 January 2015 (UTC)
@Smokefoot: That would be a valid point, except that 'real' chemists like Ben Mills have used 3D models to depict flexible molecules. To name a few: octadecyltrichlorosilane, isobutylamine, paullinic acid. I can't claim to speak for Ben, but he has said recently that "No image is better than a bad image." So logically, he couldn't possibly regard these as bad images.
Now, ideally these would be based on crystal structures (as I have recently started to do) but in reality that is not always possible. So the next best option is to take an educated guess - either through energy minimisation in DS Visualizer, or by inferring from the structure of a similar compound. This may seem to be bordering on WP:OR, but given the points discussed above (flexible molecules have more than one configuration, and all molecules are vibrating all the time), I think it is more than acceptable. For future reference though, should I add a post script to the description of my images to say that's where I got the structure from?
Jynto (talk) 17:30, 29 January 2015 (UTC)
Not only have I taken an educated guess when creating the models I submitted, but I provide citation in the form of external links to other sites which use the same configuration on each image as proof of it's acceptability as a reference NOT to disprove other configurations. I think this should be standard procedure.
@Testem: The statements that 3d images are not used 99% of the time, and that all content provided by Lazord00d is "incorrect" are only conjecture unless proven. Lazord00d (talk) 17:57, 29 January 2015 (UTC)
@Boghog: I'm having trouble understanding the grounds for your post in agreement above. It's not simply that you agree that I'm having issues with, it's the fact that your explanation suggests a more complicated process for the reader from my view. Would that not be counter-productive in a public resource? Lazord00d (talk) 19:48, 29 January 2015 (UTC)
- @Lazord00d: Quite to the contrary. What I am suggesting is a more complicated procedure for the editor to make it easier for the reader.
YourTestem's proposal quite reasonably suggests that a 3D depiction should only be included if it aids understanding. My extensionof yourTestem's proposal adds the requirement that the additional understanding be made crystal clear to the reader by supporting prose backed up by reliable sources. Boghog (talk) 21:02, 29 January 2015 (UTC)
- @Boghog: Ahh I see what you're saying. I misunderstood you, but I think you have my position confused with another editor. I'm of the belief that every molecule on WP should have 2d and 3d representation in their articles, preferably right next to each other and oriented as similarly as possible, which *should* (hopefully) allow understanding of the relationship between the two at a glance even by novices. I don't have citation for that position so it is an opinion of mine to be sure, but it's one that has proven itself repeatedly. Many if not most humans are very visual learners, which Jynto has already mentioned. That alone means that imagery is almost always helpful unless redundant or blatantly and provably incorrect.
- @Lazord00d: Corrected above. This entire thread is becoming quite confusing. Boghog (talk) 22:39, 29 January 2015 (UTC)
- @Boghog: Indeed :-).. It would be pretty sweet if WP talk pages had more of a forum appearance, ie each comment clearly separated.. Here unless people indent their posts you have to pretty much read as carefully as you can just to find the username at the end of the post so you know where to respond lol. Lazord00d (talk) 23:38, 29 January 2015 (UTC)
@Benjah-bmm27: I think your post in the poll is pretty much spot on, except that if there is to be a panel of editors to approve the placement of all 3d models based on those people's personal credentials, wouldn't it be good to include verifiable credentials in the user's data somewhere? Anyone can claim to be formally educated in a topic without that actually being the case.. Lazord00d (talk) 19:54, 29 January 2015 (UTC)
Toxin templates
Please could somebody look at the ad-hoc templates on Androctonus australis hector insect toxin and Birtoxin? Should these be replaced with {{Chembox}}, or something else? Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 16:19, 31 January 2015 (UTC)
- These toxins are proteins, not small molecules and hence more within the scope of WP:MCB than WP:CHEMS. There is already a {{infobox nonhuman protein}} in the article that would be more appropriate. Boghog (talk) 16:43, 31 January 2015 (UTC)
%vol?
Pls see Talk:Volume fraction#%vol. Thx. Fgnievinski (talk) 18:22, 31 January 2015 (UTC)
Carl Djerassi
Carl Djerassi died over the weekend. He was a big contributor to early steroid chemistry but is probably best known for helping to develop the oral contraceptive pill. His article is in pretty good shape but if anyone can think of anything that's missing now would be a good time to add it as the page is currently getting several thousand hits per day. --Project Osprey (talk) 21:26, 2 February 2015 (UTC)
Nomenclature Problem
Hello, I am aGastya. I am confused that American spellings for chemical compounds to be used or IUPAC ones? Like Sulfate vs Sulphate? aGastya 04:01, 7 February 2015 (UTC) — Preceding unsigned comment added by AgastyaC (talk • contribs)
Substrates and products merge
Just a quick post to both WP:MCB and WP:Chem. There are duplicate stub pages for substrate and product. Since in each case one copy just repeats the other I propose that they're merged into substrate_(chemistry) and product_(chemistry) with the biochem-specific parts just as a section.
Discussion on WP:MCB page. T.Shafee(Evo﹠Evo)talk 09:34, 20 January 2015 (UTC)
For reference, I've performed the merge and expanded the articles a bit. The input of any chemists would be very welcome! T.Shafee(Evo﹠Evo)talk 03:52, 10 February 2015 (UTC)
Carbohydrates
I draw attention to the deplorable state of many articles on carbohydrates. Just 3 examples from many
- pentose does not have either Fischer projections or cyclic structures
- hexose does have Fischer projections, but shows cyclic structure only for glucose and mannose
- Ribose has all the structures but none for deoxy ribose.
It's not just the content of these and other articles. The subject carbohydrate is not a good overview as it is limited to monosaccharides and disaccharides. Might I suggest that this subject area needs to be re-organized and that a lot of re-writing is also needed. Petergans (talk) 06:20, 10 February 2015 (UTC)
Nomination for deletion of Template:InChI
![]() Template:InChI has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. -- 70.51.200.101 (talk) 05:57, 11 February 2015 (UTC)
Template:InChI has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. -- 70.51.200.101 (talk) 05:57, 11 February 2015 (UTC)
More opinions on the order of the identifiers in the chembox would be appreciated. --Leyo 22:02, 17 February 2015 (UTC)
IUPAC again
I've repeatedly heard from members of this WikiProject that articles should not be forced to blindly adopt IUPAC definitions or terminology in instances where they directly contradict common use by actual scholars, researchers, etc in a particular field. And yet, it appears that we have an entire policy page mandating strict adherence to IUPAC guidelines in articles: Wikipedia:Naming conventions (chemistry). How does that work? (+)H3N-Protein\Chemist-CO2(-) 14:43, 19 February 2015 (UTC)
- See the 'exceptions' section. --Project Osprey (talk) 14:54, 19 February 2015 (UTC)
- The IUPAC guideline is specific that it applies to chemistry articles. Now within that narrowed field, do we really have articles where this is contentious? Chemists in the UK aren't going to stop spelling it "sulphur" until the current generation dies off. However they're not likely to oppose IUPAC for sake of "common use" either. Andy Dingley (talk) 15:00, 19 February 2015 (UTC)
Possibly notable chemist who died in last week's train wreck
One of the victims of last week's Valhalla train crash was Robert M. Dirks, a computational chemist with a doctorate from Caltech, where he also did a postdoc, who worked at D.E. Shaw Research in Manhattan. Since another victim, Walter Liedtke, has turned out to be notable, I decided to look into Dirks ... who seems to have coauthored quite a few papers, and his employer describes him as having made "tremendous contributions" to "the development of novel computational chemistry methods."
I've compiled what I've found on the article's talk page. I'd like some input from editors at this project who could say what's significant about his research, if it is significant, within the context of the notability criteria for academics and scientists than I am, and perhaps better qualified to judge if Dirks' research makes him notable. Daniel Case (talk) 16:41, 8 February 2015 (UTC)
- Probably not. If he were notable, his name would/will come up without prompting. PhD's are virtually required to publish papers in chemistry, so that is a low bar. CalTech cranks out about 25 PhDs each year in chemistry. He went from his PhD to an investment bank, a career path that would indicate that he was going to develop is quantitative skills, not scholarship. Tragic nonetheless.--Smokefoot (talk) 16:58, 8 February 2015 (UTC)
- Just noticed this, belatedly. Note that D. E. Shaw Research and D. E. Shaw & Co. are not the same thing; Dirks was an employee of the research group, not the hedge fund, and is an author on several of the company's papers. Nevertheless, I don't think he's notable in the Wikipedia sense. We don't have articles on any of the other company scientists as far as I can tell. Opabinia regalis (talk) 20:04, 21 February 2015 (UTC)
- I work in the same field as him (DNA nanotechnology) and I can say that his publications are immensely impactful within this field. He developed the thermodynamic analysis algorithms that underlie NUPACK, a nucleic acid design suite that is the primary tool of this kind used in the field. I was thinking of making an article about him myself, I just need to dig up some third-party sources that aren't obituaries. I'm happy to work on it with you, Daniel Case, if you're still interested. Antony–22 (talk⁄contribs) 01:14, 22 February 2015 (UTC)
- Just noticed this, belatedly. Note that D. E. Shaw Research and D. E. Shaw & Co. are not the same thing; Dirks was an employee of the research group, not the hedge fund, and is an author on several of the company's papers. Nevertheless, I don't think he's notable in the Wikipedia sense. We don't have articles on any of the other company scientists as far as I can tell. Opabinia regalis (talk) 20:04, 21 February 2015 (UTC)
- Fair enough, I was looking at the simulation side of things. Biographies of the marginally notable and recently deceased worry me a little - they're unlikely to be actively watched in the long term - but no objections. Opabinia regalis (talk) 05:26, 22 February 2015 (UTC)
Anyone heard of "Groove's process"?
Yesterday I started this AfD for the articles groove synthesis and groove halogenization, which are clunky descriptions of the synthesis of haloalkanes from alcohols. I couldn't come up with any connection between these names and the reaction, but I did find a handful of references to "Groove's process" as a term for it. It does seem to be something students are searching for, but I can't find anything to substantiate the name. Anyone heard of this? Opabinia regalis (talk) 05:33, 22 February 2015 (UTC)
- In general a very simple transformation which is also a named reaction tends to be a very old named reaction - and some of those names just fall out of use. The Menshutkin reaction is now generally just called quaterisation, no one really calls the Darzens halogenation anything anymore. In this case I would have thought that Lucas' reagent would have covered everything we need. --Project Osprey (talk) 09:50, 22 February 2015 (UTC)
- Thanks - yeah, I know these are often just obsolete names, but Darzens halogenation et al. are names that can be found in typical English-language organic chemistry references even if they're not really used anymore. "Groove's process" doesn't appear in equivalent places, but does seem to be search term (used mostly by Indian students, it looks like). I wondered if anyone outside of homework-help sites had actually run across that usage that would warrant a redirect. Opabinia regalis (talk) 21:26, 22 February 2015 (UTC)
- Well I'm in work tomorrow and my access to literature will be much better, so I'll do a little digging and post again if I find anything. --Project Osprey (talk) 21:39, 22 February 2015 (UTC)
- There is absolutely nothing in the normal scientific literature. The best I could find were two references to it in text books "The Pearson Guide To Organic Chemistry For The Iit Jee" and "Together With Iit-Jee Chemistry", LIT JEE being a university entrance exam in India. I can only look at one of those via Google Books (The Pearson Guide) and it's a bit weird. There are spelling errors ("MarkownoKoff's rule" instead of Markovnikov's rule) and strange re-inventing's of chemistry (The obscure Rydon Reagents are now the Rydone process: first equation below is what the book said happens, the second is what should actually be shown)
- Well I'm in work tomorrow and my access to literature will be much better, so I'll do a little digging and post again if I find anything. --Project Osprey (talk) 21:39, 22 February 2015 (UTC)
- Thanks - yeah, I know these are often just obsolete names, but Darzens halogenation et al. are names that can be found in typical English-language organic chemistry references even if they're not really used anymore. "Groove's process" doesn't appear in equivalent places, but does seem to be search term (used mostly by Indian students, it looks like). I wondered if anyone outside of homework-help sites had actually run across that usage that would warrant a redirect. Opabinia regalis (talk) 21:26, 22 February 2015 (UTC)
- R-CH2OH + Br2 + (C6H5O)3PO → R-CH2Br + C6H5OH + (C6H5O)2POBr
- (C6H5O)3P + MeI → (C6H5O)3P+Me I-
- (C6H5O)3P+Me I- + ROH → (C6H5O)2P+(Me)OR I- + C6H5OH → (C6H5O)2P(O)Me + RI
- Most of the information in the book looks accurate but there's enough strange content to stop me from trusting it. By extension I don't think that the "Groove's process" really exists (at least in terms of it being named after a Dr Groove), I think we can safely delete those pages or at least tern them into redirects. --Project Osprey (talk) 18:08, 23 February 2015 (UTC)
- Thanks, glad I'm not the only one who thinks this is strange! I think the only title with any basis for a redirect would be Groove's process, since the other variants turn up basically nothing. But the existence of a redirect sort of implicitly acknowledges the existence of the name - it's harmless enough but in general neologisms like this whose only source is clearly unreliable don't survive here. Opabinia regalis (talk) 05:27, 24 February 2015 (UTC)
- Most of the information in the book looks accurate but there's enough strange content to stop me from trusting it. By extension I don't think that the "Groove's process" really exists (at least in terms of it being named after a Dr Groove), I think we can safely delete those pages or at least tern them into redirects. --Project Osprey (talk) 18:08, 23 February 2015 (UTC)
- Some time ago I cleaned up the List_of_organic_reactions here but since then people have been adding reactions (often obscure ones) back in V8rik (talk) 21:05, 24 February 2015 (UTC)
I could use some input about the relatively new article N-Acylamides. The article is basically about fatty acid amides and I think it should be merged into that article. But the article content has some problems I would like to address before I merge it. First of all, the writer seems to be unclear about the difference between an amine and an amide (which leads to the bad nomenclature in the title, to begin with). I'm not sure I trust content written by someone without enough knowledge of the topic to understand the distinction between those two functional groups. Setting aside the nomenclature problems, does the rest of the content hold up to scrutiny? ChemNerd (talk) 20:10, 3 March 2015 (UTC)
- Looks very suspect. --Smokefoot (talk) 23:08, 3 March 2015 (UTC)
- Looks like a confused student effort? The description is bad and the glycerols don't belong, but the term "N-acyl amide" does get used in the literature sometimes to refer to a fatty acid attached to an amino acid or ethanolamine. I think it's part of the general terminological muddle on what to call N-acylethanolamines. Most of these are endogenous cannabinoids involved in lipid signaling. Opabinia regalis (talk) 05:03, 4 March 2015 (UTC)
- Some of the entries in the article's table are n-acylamino acids (e.g., N-acyl glycine) which are structurally distinct from fatty acid amides. They should be split out into a new article or deleted. Boghog (talk) 05:39, 4 March 2015 (UTC)
- Looks like a confused student effort? The description is bad and the glycerols don't belong, but the term "N-acyl amide" does get used in the literature sometimes to refer to a fatty acid attached to an amino acid or ethanolamine. I think it's part of the general terminological muddle on what to call N-acylethanolamines. Most of these are endogenous cannabinoids involved in lipid signaling. Opabinia regalis (talk) 05:03, 4 March 2015 (UTC)
"Binary liquid" notability
Is "binary liquid" an actual notable term, that doesn't just mean "two liquids mixed together"? The only source I can find that refers to it as something distinct is the Wikipedia page. Jay Vogler (talk) 21:27, 1 March 2015 (UTC)
- I see that the first and third external links of binary liquid lead to the same article, which does not contain the word "binary" and is about foam; while the second gives a "not found" error. It looks to me like a poorly constructed hoax article. Maproom (talk) 08:13, 2 March 2015 (UTC)
- In Google Scholar searching for "binary liquid" nets 38,100 hits; the general concept seems notable enough. Binary liquid as "two inert liquids, that when mixed together, do something interesting" seems much less so, but binary liquid explosives have been discussed as possible/improbable terrorist devices, e.g., flying toilet terror labs. --Mark viking (talk) 11:59, 2 March 2015 (UTC)
- All those mentions seem to be "binary" modifying some type of liquid composition, to note that it's two parts; nowhere do I see "binary liquid" on its own as a notable thing. In the case of a binary liquid explosive it's not the term binary liquid which is of interest, but a liquid explosive which happens to be binary, and I'd argue information on that fits much better into binary explosive, maybe binary liquid explosive. This looks a lot like someone made up their own specialized definition and tossed it into Wikipedia for kicks. Jay Vogler (talk) 14:19, 4 March 2015 (UTC)
- Actually, I take part of it back, study of the properties of binary liquid mixtures seems to be a thing. It still has nothing to do with the definition as currently written, though, and I'm not sure whether it deserves its own page any more than binary amalgam or binary gas, etcetera, do. What could be said about the idea beyond a dictionary definition? Jay Vogler (talk) 14:20, 4 March 2015 (UTC)
- All those mentions seem to be "binary" modifying some type of liquid composition, to note that it's two parts; nowhere do I see "binary liquid" on its own as a notable thing. In the case of a binary liquid explosive it's not the term binary liquid which is of interest, but a liquid explosive which happens to be binary, and I'd argue information on that fits much better into binary explosive, maybe binary liquid explosive. This looks a lot like someone made up their own specialized definition and tossed it into Wikipedia for kicks. Jay Vogler (talk) 14:19, 4 March 2015 (UTC)
The Merck Index: updating citations
My Royal Society of Chemistry colleagues and I propose to update citations to The Merck Index. As there may be CoI concerns, please review the proposal, and comment, at Wikipedia:GLAM/Royal Society of Chemistry/Merck. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 15:50, 4 March 2015 (UTC)
FAR
I have nominated Aldol reaction for a featured article review here. Please join the discussion on whether this article meets featured article criteria. Articles are typically reviewed for two weeks. If substantial concerns are not addressed during the review period, the article will be moved to the Featured Article Removal Candidates list for a further period, where editors may declare "Keep" or "Delist" the article's featured status. The instructions for the review process are here.--Jarodalien (talk) 14:52, 7 March 2015 (UTC)
Hello again chemistry experts. I'm not sure if this is the best place to report this draft, but perhaps someone here can tell me if this is a notable topic, and if the sources are appropriate. If it is accepted, it would fill in a red link in this page: Primary production. —Anne Delong (talk) 05:29, 23 May 2015 (UTC)
AfC submission
What are your thoughts on Draft:Synthesis using Regiospecific Enolate Formation and Masked Functionality? Thanks, FoCuSandLeArN (talk) 02:45, 21 May 2015 (UTC)
- Also Draft:Thermal rearrangements of aromatic hydrocarbons, Draft:Modified Aldol Tandem Reaction and Draft:Substrate control: asymmetric induction by molecular framework in acyclic systems. FoCuSandLeArN (talk) 15:15, 21 May 2015 (UTC)
- I have accepted Thermal rearrangement of aromatic hydrocarbons, and I think Smokefoot has a good suggestion to merge the Draft:Modified Aldol Tandem Reaction only. I cannot find any evidence that the title has been used by any other person than the draft writer. The last one could be merged to Asymmetric induction. Graeme Bartlett (talk) 02:17, 22 May 2015 (UTC)
New round of school-project articles
Education Program:Mahidol University International College/ICCH224 (T3/2014-2015) DMacks (talk) 16:06, 15 May 2015 (UTC)
The Merck Index Online
Quick straw poll: how many of you have access to The Merck Index Online (perhaps though work or academic institution)? Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 15:24, 27 April 2015 (UTC)
- I do, do you need something in particular? Emily Temple-Wood (NIOSH) (talk) 22:43, 27 April 2015 (UTC)
Chemistry societies navbox
I have created {{Chemistry societies}}; it should be deployed to each of the articles it lists, shortly. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 09:43, 21 April 2015 (UTC)
- Great. We need an article on the Federation of Asian Chemical Societies (FACS). There are plenty of articles that mention it. It would then be added to this navbox. --Bduke (Discussion) 10:03, 21 April 2015 (UTC)
- Indeed. I've added that to the (long) list of red links for chemistry societies, at Wikipedia:GLAM/Royal Society of Chemistry#Organisations. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 10:50, 21 April 2015 (UTC)
Who?
Which editor posted that serious question I'd like to reply to? Was it at Chemistry, Chemicals, Chembox, Drugbox, Chem infobox, xxx? -DePiep (talk) 22:15, 12 April 2015 (UTC)
drugfuture.com
We currently have a bunch of links to drugfuture.com (see [1]). I think this website is just hosting content stolen from elsewhere such as Merck Index. Can anyone who has access to Merck Index please check? If the website is hosting copyrighted material, since Wikipedia:Copyrights says, "if you know or reasonably suspect that an external Web site is carrying a work in violation of the creator's copyright, do not link to that copy of the work", we should either retarget the links to the original source or remove the links. ChemNerd (talk) 17:25, 13 April 2015 (UTC)
Wikipedia session at ACS Boston meeting in August
The ACS Chemical Information division (CINF) is holding a technical session at the Fall ACS meeting in Boston, titled "Wikipedia and Chemistry: Collaborations in Science and Education". We're trying to highlight the contribution of Wikipedia to chemistry and to chemical education. If you have a suitable topic you'd like to present on, and you'd like to submit an abstract, submission is open until March 29th. If you have questions, by all means contact me on my talk page. Walkerma (talk) 04:23, 16 February 2015 (UTC)
- This is just a quick followup to remind people - abstracts are due by this coming Sunday. I see I didn't post the full description before, which is:
Chemistry information on Wikipedia has an enormous reach and impact. In this symposium, the connections between Wikipedia editors and the wider chemistry community will be explored. In particular, collaborations between educators, students and regular editors (Wikipedians) will be examined to understand how Wikipedia editing can be set up as a successful class project. Educators will demonstrate how Wikipedia projects can help students with understanding subject matters and improving information literacy skills. Meanwhile, we will explore how educators and the Wikipedia community can work together through the Wikipedia Education Program to ensure students' contributions to Wikipedia are valid and effective. Finally, we will exchange ideas on how to involve more chemists in contributing to Wikipedia, and how to use the site wisely.
- We are also organising an Edit-a-thon, so even if you're not presenting, we'd love for you to help with that. Thanks, Walkerma (talk) 20:25, 27 March 2015 (UTC)
The usage of Carbon fiber (edit | talk | history | protect | delete | links | watch | logs | views) is under discussion, see talk:carbon (fiber) -- 65.94.43.89 (talk) 05:06, 22 March 2015 (UTC)
RFC: Acronym NMR or nuclear magnetic resonance in article titles
The following discussion is closed. Please do not modify it. Subsequent comments should be made on the appropriate discussion page. No further edits should be made to this discussion.
| Nuclear magnetic resonance (NMR) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core concepts | |||||||||||||||
|
|||||||||||||||
| By isotope | |||||||||||||||
|
Article titles in Category:Nuclear magnetic resonance are a mix of spelled out (e.g. nuclear magnetic resonance spectroscopy) and acronym (e.g. gradient enhanced NMR spectroscopy). The Manual of Style WP:ACRONYMTITLE says that "acronyms should be used in a page name if the subject is known primarily by its abbreviation and that abbreviation is primarily associated with the subject" and "if readers somewhat familiar with the subject are likely to only recognize the name by its acronym". Should NMR articles always use "NMR", always use "nuclear magnetic resonance", or should use be determined on a case-by-case basis? If case-by-case, what criteria should be used? --Kkmurray (talk) 22:28, 22 March 2015 (UTC)
- Whilst I don't have a strong opinion either way right now, I feel that anyone "somewhat familiar with the subject" would recognize NMR as being nuclear magnetic resonance. It is also worth noting that NMR redirects to nuclear magnetic resonance as well. Lukeno94 (tell Luke off here) 23:11, 22 March 2015 (UTC)
- I'm guessing that the idea behind this is that people are searching for these pages but that few users are going to bother typing 'nuclear magnetic resonance' each time. So yes, seen through that lens page titles should be contracted to NMR where possible. I'm not sure how much that's going help people though; by necessity many of these pages have long and technical titles which could be phrased in a number of different ways, so people might still have difficulty finding what they want. I would suggest that a navigation sidebar might be more helpful, I've attached a draft of what one might look like. Anyone got any thoughts? --Project Osprey (talk) 10:48, 23 March 2015 (UTC)
- NMR. I favor the acronym for simplicity of typing and because it is routinely used and one rarely spells out the acronym. We probably want to have uniformity for the titles of these articles. At least in my circle, we dont say MS for mass spectrum but for NMR, we avoid enunciating those 9 syllables.--Smokefoot (talk) 13:19, 23 March 2015 (UTC)
- I’ll give my own comment here: In general I prefer non-acronym titles - NMR should be spelled out in titles but doesn't need to be spelled out on first use, similar to FM and PC (but note acronyms in FM broadcasting and IBM PC keyboard!). The bar for acronym titles should be high (e.g. DNA, laser, sonar). The current set of redirects takes care of searches and acronym use in articles. That said, there may be a limited number of cases where the acronym makes more sense, for example NMR tube which is not recognizable as nuclear magnetic resonance tube. Another case might be the DAB, as in relaxation (NMR). --Kkmurray (talk) 01:47, 24 March 2015 (UTC)
- NMR for sure. Shorter and easier to type; no other similar acronyms in the field to cause confusion; readers of most of these articles almost surely know what NMR is and don't need it spelled out. Opabinia regalis (talk) 03:53, 24 March 2015 (UTC)
- write out - Reasoning: I tried to see what news sources on internet tend to use, but Google does not really give me a good count on that. I do notice that many news articles do a 'nuclear magnetic resonance (NMR)' in the title or early text, and from then on use NMR. This discussion gets in the direction of 'EEG', 'CPR', 'MRI' and 'ECG' - where the man in the street probably hardly know what these medical abbreviations stand for (and personally I would probably make typos trying to write it out ..), though they know what these techniques do. I see that the medical articles tend to write out the whole all the time, so I guess that I would go for the written out term 'nuclear magnetic resonance' in the article titles and introduction (and immediately introducing the abbreviation NMR as well as after the first wikilink to nuclear magnetic resonance ('Nuclear magnetic resonance (NMR)'), followed by NMR in the remainder of the text. That would then also go in line with MS, E.A. and IR (both for consistency throughout our articles on experimental techniques which are not as strictly pronounced as for NMR) .. For all article titles, the appropriate abbreviated articles need to exist. --Dirk Beetstra T C 05:34, 24 March 2015 (UTC)
- NMR first I think a lot of students (High school) wouldnt recognize nuclear magnetic resonance, second in science articles (also titles) its normally just NMR, and third, Im not sure I would recognize that the article paramagnetic nuclear magnetic resonance spectroscopy was about NMR (all the references in the article use NMR - the article has been moved recently). Christian75 (talk) 14:00, 25 March 2015 (UTC)
- NMR but identify the acronym sometime, somewhere in the article. When I graduated with an undergraduate degree in in Chemistry in 1980, we did not use the acronym...but time changes things. Pretty soon no one will even know what the acronym stands for. Best Regards, Bfpage |leave a message 22:18, 28 March 2015 (UTC)
- NRMIt agrees with the manual of style, and is a lot simpler than having to type it out all the time. It should probably say what NRM means somewhere in the article. Rider ranger47 Talk 00:28, 9 April 2015 (UTC)
- I am humble to suggest that full version "Nuclear magnetic resonance" is the one that should be used anywhere and everywhere. My argument is that WP is not to make the things simple for the convenience of people, WP is just an encyclopedia to present the things as they are for all sort of people (expert/non expert) who search the encyclopedia. I agree that most of us, discussing here know well in advance what NMR means in science, but a non-expert would have difficulty to guess. In my view, He should be presented with an info that is precise, full and authentic. I just give an example, have you ever see the prescription slips of the doctors? They dont write the name of medicines in full, and sometime used just some symbols that only a chemist can understand. In due course, their entire community became familiar with those codes, symbols and acronyms etc. For them, it is a routine and probably "Easy" to write the things that way. Just imagine if there is an article on wiki titled "MS Adv++" what one will guess from this? But doctors know that it may either be Multiple Sclerosis in ++ stage, or Marphans Syndrome with ++ abnormalities and so on. A doctor is much happier with this acronym but the same doctor may not even know the meaning of NMR, which is a common term for a researcher/scientist. Thus, I suggest that let it be authentic name and not the one that has become popular in due course or has become known to the researchers in dues course; we have to be friendly with all sort of readers. yes, a seperate explaination page can be created where all possible meaning of NMR (in use at present in various areas) could be placed and let the user (who is only aware of some NMR and not its full version) distribute from there to appropriate article as per his need. And yes, after the first use of full version in title, and in lead, the short version can be introduced, when it appears first in text - that may continue for rest of the article. Educationtemple (talk) 07:29, 13 April 2015 (UTC)
- Spell it out - acronyms are best reserved for things known primarily by the acronym. The bar for this is set quite high, as is the case for DNA, Nato, Laser etc etc. As for related article titles, there should be a case by case discussion to weight out consistency with other considerations in regards to article title. Mbcap (talk) 14:44, 20 April 2015 (UTC)
Changing Molecular formula to Chemical formula in chembox
I suggest changing "Molecular formula" to "Chemical formula" in {{Chembox Formula}} (the chembox), it is used for salts too - like Sodium chloride. Right now, its a piped redirect to chemical formula e.g. [[Chemical formula|Molecular formula]] which I propose should be changed to [[Chemical formula]]. There is one objection aginst this change, please comment at Wikipedia_talk:Chemical_infobox#Changing_Molecular_formula_to_Chemical_formula Christian75 (talk) 17:26, 30 March 2015 (UTC)
If anyone could help with this article, I'd be grateful. It's been tagged for notability, and many other issues, for 7 years. Best wishes, Boleyn (talk) 08:03, 31 March 2015 (UTC)
Astatine FAC
Hi! The article astatine has been at FAC for quite some time now, and the process could really use some more input. If you could take some time to come in, please give it a look. Unfortunately, the daughter WP:ELEMENTS is very small and can't really organize a high number of editors who could come in. Any effort, positive or negative, would be much appreciated, and, if you wish, a favor in return is on me.--R8R (talk) 10:02, 6 April 2015 (UTC)
suggested COI edits at Dow Corning
Hi, I've proposed some additions to Dow Corning on the article's Talk page, here. I'm not editing directly because I have a COI (I work for a communications firm that represents Dow Corning), but if anyone here is interested in taking a look and providing feedback, I'd be really grateful. Thank you! Mary Gaulke (talk) 16:28, 20 April 2015 (UTC)
- Thanks for being upfront and going about this the right way. --99of9 (talk) 22:26, 24 April 2015 (UTC)
Does anyone want to assist with finishing and polishing an already DYK appropriate article?
I started working on User:Ryan Vesey/Xerotine siccative in mid 2011 (Xerotine siccative was a paint thinner that caused explosions on a number of ships in the late 19th century) and have never been able to finish the article. It meets the DYK requirements already, but I really want to have an article that is both polished and as complete as possible before I submit it. I haven't been active much in the past 3 years and don't know if I'll ever find the time to finish it myself, but I hate to see it sit in my user space, unseen by anybody. Is there anyone here who is interested in taking up the project? I don't believe I ever fully got through the House of Commons papers that I used as a source, so those should provide a good amount of information. I'm leaving messages at WikiProject Ships and WikiProject History as well, since editors there may have an interest. Ryan Vesey 20:27 (UTC), May 15, 2015