Wikipedia:Reference desk/Archives/Science/2019 July 30
| Science desk | ||
|---|---|---|
| < July 29 | << Jun | July | Aug >> | Current desk > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
July 30
[edit]What if Antarcica had no land
[edit]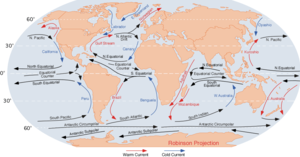
What if Antarcica had no land but just a deep ocean? Would there be a giant iceberg there, could it float away?
- Where would it go? There's an Arctic ice cap as well, just ice sitting in an ocean, but it doesn't float away; it doesn't really change, aside from growing and shrinking in the winter and summer. Part of the issue probably has to do with ocean currents; as you can see from the map at right, there's a weaker current circling Antarctica going west, and a stronger current circling Antarctica going east. A continent-sized ice pack presumably would be influenced by the currents equally on all sides, so I suppose (can't prove this) that this reduces the likelihood of its breaking loose, and if it did break loose, the currents would incline it to spin. Can someone more knowledgeable confirm that this is correct about the currents, or explain where I've gone wrong? Nyttend (talk) 10:41, 30 July 2019 (UTC)
- Currents would break off those projections on the map first, if not anchored by land. Ice shelves (or portions) have broken off, some quite large (Wordie Ice Shelf, Jones Ice Shelf, Müller Ice Shelf, Prince Gustav Ice Shelf, Larsen Ice Shelf). After they break off they float away, break up, and eventually melt ([1]). The land is quite important, in two respects, to prevent the ice from melting. First, it anchors the ice at the coldest part of the planet, preventing it from moving to warmer areas and melting. Also, since the ice is freshwater, it will stay frozen at temps up to 32F, but once in the ocean, and exposed to saltwater, it can melt at lower temps, down to
028.4F. There is much less ice at the North Pole than at the South Pole, and this is due to the presence of land in Antarctica. All that meltwater would increase water levels substantially (61 meters/200 feet [2]). However, if the space currently occupied by land in Antarctica, below the waterline, was filled in with water, that would lower ocean levels perhaps even more. Also the water there then absorbing sunlight that is currently reflected back into space by ice would accelerate global warming. Without land, thin ice would form near the South Pole every winter, in any case, but some, or perhaps all, would melt in the summer, similar to the pattern we see at the North Pole (note that summer and winter are reversed between the North and South hemispheres). SinisterLefty (talk) 13:19, 30 July 2019 (UTC)
- Currents would break off those projections on the map first, if not anchored by land. Ice shelves (or portions) have broken off, some quite large (Wordie Ice Shelf, Jones Ice Shelf, Müller Ice Shelf, Prince Gustav Ice Shelf, Larsen Ice Shelf). After they break off they float away, break up, and eventually melt ([1]). The land is quite important, in two respects, to prevent the ice from melting. First, it anchors the ice at the coldest part of the planet, preventing it from moving to warmer areas and melting. Also, since the ice is freshwater, it will stay frozen at temps up to 32F, but once in the ocean, and exposed to saltwater, it can melt at lower temps, down to
- You have to add NaCl till more would just precipitate no matter how much you stir for it to not freeze till 0. Seawater is only 10% saturated with salt and freezes at 28. Sagittarian Milky Way (talk) 17:07, 30 July 2019 (UTC)
- Thanks. Updated my post. I should also add that convection occurs in the ocean, carrying away the cold, just melted water, and bringing in warmer water to melt the rest. SinisterLefty (talk) 17:39, 30 July 2019 (UTC)
- I am afraid nobody can give the answer, nobody understand the Earth system as it currently is, understanding how id would be is just impossible. :The simplest way to imagine this is: it would behave just like the arctic Gem fr (talk) 15:27, 30 July 2019 (UTC)
- Except the Arctic Ocean is mostly surrounded by land, and Antarctica by water. Take away Antarctica and you have an enlarged Southern Ocean that would still be contiguous with the Pacific, Atlantic, and Indian Oceans. --76.71.6.164 (talk) 16:03, 30 July 2019 (UTC)
- well, in this scenario, the land currently in Antarctica could be used to fill the Arctic. problem solved Gem fr (talk) 19:28, 30 July 2019 (UTC)
- Except the Arctic Ocean is mostly surrounded by land, and Antarctica by water. Take away Antarctica and you have an enlarged Southern Ocean that would still be contiguous with the Pacific, Atlantic, and Indian Oceans. --76.71.6.164 (talk) 16:03, 30 July 2019 (UTC)
- Without Antarctica at the South pole there could at most be far less ice at the South pole, but this means that far more solar radiation would be absorbed there. It would thus be a lot warmer on Earth, we would likely not be in an ice age today (we're now in a short interglacial period in-between the longer glacial phases of the ice age). So, there wouldn't be any ice at the North Pole and on Greenland either. Count Iblis (talk) 21:23, 30 July 2019 (UTC)
- How do we know that the ice age hasn't now ended ? SinisterLefty (talk) 12:16, 3 August 2019 (UTC)
- Have all the glaciers melted yet? ←Baseball Bugs What's up, Doc? carrots→ 12:32, 3 August 2019 (UTC)
- How do we know that the ice age hasn't now ended ? SinisterLefty (talk) 12:16, 3 August 2019 (UTC)
- Let me rephrase as "How do we know we aren't nearing the end of the ice age, as opposed to being in an interglacial period ?" SinisterLefty (talk) 12:54, 3 August 2019 (UTC)
- On the plus side, there would no longer be such a danger from sea level rise due to global warming. (If floating ice melts, it doesn't change the sea level.) --76.71.6.164 (talk) 06:37, 3 August 2019 (UTC)
- That would depend on what we replace the missing land with when we dematerialize Antarctica (and to what depth). If we replaced it with air or a vacuum, say 5 miles deep, then, once the massive tsunamis and atmospheric shock waves subsided, sea level would be much lower. If we replaced it with water, then the ice on top of what was Antarctica would plunge into the water and raise ocean levels substantially. In any case, there would be the secondary effect mentioned by Count Iblis, and all the ice on Greenland melting and other glaciers would still raise sea levels (although nowhere near as much as it would drop in the first scenario). SinisterLefty (talk) 12:16, 3 August 2019 (UTC)
- A more realistic question might be, if the entire land mass had drifted from the south pole to where Australia is now, instead of just half of it, what might the south polar region look like? ←Baseball Bugs What's up, Doc? carrots→ 12:32, 3 August 2019 (UTC)
- That would depend on what we replace the missing land with when we dematerialize Antarctica (and to what depth). If we replaced it with air or a vacuum, say 5 miles deep, then, once the massive tsunamis and atmospheric shock waves subsided, sea level would be much lower. If we replaced it with water, then the ice on top of what was Antarctica would plunge into the water and raise ocean levels substantially. In any case, there would be the secondary effect mentioned by Count Iblis, and all the ice on Greenland melting and other glaciers would still raise sea levels (although nowhere near as much as it would drop in the first scenario). SinisterLefty (talk) 12:16, 3 August 2019 (UTC)
- So Florida would already be under water, but we wouldn't notice it, because it never would have been settled. ←Baseball Bugs What's up, Doc? carrots→ 11:21, 3 August 2019 (UTC)
Flatulence jet
[edit]In theory, per Newton's third law how strong should a flatulence jet be in order to lift off a person of a given weight (assuming lying horizontally rather stan standing)? And how strong for sustained flight of a given time? Inspired by Boogerman: A Pick and Flick Adventure 212.180.235.46 (talk) 08:12, 30 July 2019 (UTC)
- For someone lying horizontally (and presumably supine), the 'jet' will be significantly offset from the body's centre of mass, and will also be directed close to horizontal, so the proposed scenario is unworkable. {The poster formerly known as 87.81.230.195} 2.123.24.56 (talk) 12:16, 30 July 2019 (UTC)
- And how about sports runners? How strong a jet should be to result in their measurable acceleration? 212.180.235.46 (talk) 13:25, 30 July 2019 (UTC)
- It's never going to make a significant difference, because the density of gas, and hence the mass, is so much lower than that of a person. SinisterLefty (talk) 13:42, 30 July 2019 (UTC)
- With enough ejection speed, as small a mass as you want will do. Gem fr (talk) 15:01, 30 July 2019 (UTC)
- You will fin the formula in Thrust :
- Where T is the thrust generated (force), is the rate of change of mass with respect to time (mass flow rate of exhaust), and v is the speed of the "exhaust gases" measured relative to the "rocket".
- This means that, for instance, if you want a 900 N force (to lift a 90kg person), an ejection of 1 gram per second need to have a speed of 900,000 m/s . This is
3%(my bad) 0.3% of light speed (quite a feat, but not impossible), but x3000 the speed of sound, so expect some supersonic boom Gem fr (talk) 15:01, 30 July 2019 (UTC) - Actually 900,000 m/s = 0.3% of light speed, a feat involving a significant contraction, even for Hendrik Lorentz. It's 26,000 times the speed of sound in air. DroneB (talk) 15:30, 30 July 2019 (UTC) Actually 2,600 ;-) Gem fr (talk) 19:07, 30 July 2019 (UTC)
- May I suggest that A. this is best asked on the mathematics desk and that B. one would probably need enough thrust to "crack the bowl". Thanks for a hilarious question, not enough humour is seen on the ref desk. Anton 81.131.40.58 (talk) 16:20, 30 July 2019 (UTC)
- I wonder if a system could be devised to use flatulence for propulsion in outer space, during an EVA. A special suit would be needed to let the flatulence out without lowering the pressure in the suit. A sealed pelvic area with a valve that only opens with a given pressure differential would do it. I expect movement would be extremely slow, but eventually noticeable, so long as there was no contact with anything else (which would cause far more change in velocity) or objects nearby (their gravitational attraction may approximately equal the flatulence propulsion). See here for a discussion. They calculate 0.00012 kg m/sec, which would mean a 50 kg person would move at 0.0000024 m/sec. Make it 10 farts, and we are up to 0.000024. Multiply by an hour (3600 seconds), and 100 to convert to cm, and we have 8.64 cm/hr. Might make for a good comedy short, using time-lapse photography. SinisterLefty (talk) 12:26, 31 July 2019 (UTC)
- Regarding the above great idea, of course without the appropriate vent being implemented it would be as welcome as a fart in a spacesuit. Anton 81.131.40.58 (talk) 16:29, 31 July 2019 (UTC)
- That idea is also discussed here: [3] CodeTalker (talk) 23:45, 1 August 2019 (UTC)
Chemistry of the home-made water carbonating process
[edit]Hi, could anyone help me? I'm not british so probably I will do many grammar errors.
I have the carbonator for tap water, but instead of those little cylinders, I had it connected to a 3-liter CO2 cylinder with a valve on it (it is a very normal thing made by many). The problem is if I accidentally leave the valve open, when I gas the water I see that the CO2 enters at high pressure (I can hear a whistle and a ‘’fog’’ of CO2 appears up the surface of water in the bottle); then I get a water that is not very fizzy, and his taste and his smell are strange and bitter.
Chemically, what happened in the water when I carbonate with high pressure CO2? Why it is not fizzy and has the strange taste? --95.252.33.178 (talk) 09:06, 30 July 2019 (UTC)
- No grammar errors; the only things I'd say differently are "up the surface" (I think I'd say "on the surface" or "above the surface") and "its" instead of "his" in your last sentence. I think your English is better than you think it is :-) Nyttend (talk) 10:44, 30 July 2019 (UTC)
- When highly compressed gas depressurizes rapidly the temperature falls and a condensed phase forms, possibly dry ice with carbon dioxide. However it may just be condensing the water vapour to make water fog. You would want to have food grade carbon dioxide as contaminants in it (such as oil) might have a bad taste. But if you have enough high pressure carbon dioxide at liquid water temperatures it would make very fizzy water. Perhaps your gas cylinder does not have CO2 in it. It would be good if we had an aqueous carbon dioxide article, but I have not written it yet, an nor has any one else. Graeme Bartlett (talk) 12:47, 30 July 2019 (UTC)
- We do have articles on aqueous carbon dioxide; it is called carbonic acid or carbonated water, depending on the context. The former deals with the chemistry of aqueous carbon dioxide solutions, while the latter deals with culinary implications of such solutions. If I were to create a redirect from your red link, I would probably link to carbonic acid, since speaking of "aqueous" solutions is usually done in a chemical context. --Jayron32 13:10, 30 July 2019 (UTC)
- Maybe it could redirect to Carbonic acid as that does contain some of the chemistry, but carbonic acid is only a small component in the system of carbon dioxide in water. Graeme Bartlett (talk) 13:19, 30 July 2019 (UTC)
- That's broadly true of any acid. One could similarly say that acetic acid is only a small component of the system of hydrogen acetate in water, or that phosphoric acid is only a small component of the system of trihydrogen phosphate in water. That's how aqueous acids (or any such weak aqueous equilibrium) system works. Working out the exact concentration of each species of an acid dissolved in water is typical first-year-chemistry-major stuff. It isn't particularly worth singling out for carbonic acid, since being an aqueous acid, it's equally (and thus unremarkably) true of any other such acid, and doesn't bear special discussion. --Jayron32 15:44, 30 July 2019 (UTC)
- With the great interest in extra carbon dioxide in the atmosphere, and its effect on oceans, actually many people are interested in this in detail. Graeme Bartlett (talk) 22:32, 30 July 2019 (UTC)
- I've never said they weren't. Which is probably also why Wikipedia has articles titled Carbon dioxide in Earth's atmosphere and Oceanic carbon cycle. --Jayron32 12:38, 31 July 2019 (UTC)
- With the great interest in extra carbon dioxide in the atmosphere, and its effect on oceans, actually many people are interested in this in detail. Graeme Bartlett (talk) 22:32, 30 July 2019 (UTC)
- That's broadly true of any acid. One could similarly say that acetic acid is only a small component of the system of hydrogen acetate in water, or that phosphoric acid is only a small component of the system of trihydrogen phosphate in water. That's how aqueous acids (or any such weak aqueous equilibrium) system works. Working out the exact concentration of each species of an acid dissolved in water is typical first-year-chemistry-major stuff. It isn't particularly worth singling out for carbonic acid, since being an aqueous acid, it's equally (and thus unremarkably) true of any other such acid, and doesn't bear special discussion. --Jayron32 15:44, 30 July 2019 (UTC)
- Maybe it could redirect to Carbonic acid as that does contain some of the chemistry, but carbonic acid is only a small component in the system of carbon dioxide in water. Graeme Bartlett (talk) 13:19, 30 July 2019 (UTC)
- We do have articles on aqueous carbon dioxide; it is called carbonic acid or carbonated water, depending on the context. The former deals with the chemistry of aqueous carbon dioxide solutions, while the latter deals with culinary implications of such solutions. If I were to create a redirect from your red link, I would probably link to carbonic acid, since speaking of "aqueous" solutions is usually done in a chemical context. --Jayron32 13:10, 30 July 2019 (UTC)
- You could expect carbonic acid to form in the water, which that has more of a sour taste than bitter, but, as English is not your native language, you may have confused the two. Sour means like lemon juice, while bitter means like lemon peel. If it really tastes bitter, then I agree that it sounds like there is some contamination in the carbon dioxide bottle or equipment. It could be dangerous, so you should have it fixed/replaced. SinisterLefty (talk) 13:03, 30 July 2019 (UTC)
- No, any confusion. Is really bitter, not sour. and I'm 100% sure that in the tank there is CO2 and not another gas. I don't think there is a contamination, because I use 3-liters cylinders from years, and I change the cylinders very much times. If I open and close regularly the valve, there isn't very high pressure (in the tube which connect the cylinder and the carbonator) and the water carbonate regularly, without strange tastes. The bitter (Rhubarb, coffee, chicory are bitter) taste appears only when I leave the valve open, and there is high pressure on the tube. Is possible that at high pressure the dissolution of CO2 increase and at the same time the free CO2 (who make the fizz)decrease, obtaining a less-fizzy water but with more CO2 dissolved (so more bitter)? Thank you at all--95.252.33.178 (talk) 15:15, 30 July 2019 (UTC)
- In very simple terms, the human tongue detects low pH as sour, and high pH as bitter, so the presence of bitter flavors indicates something of a high pH. If dissolved in water directly, both the hydrogen carbonate (aka bicarbonate) and carbonate ion will raise the pH and create a bitter taste (try tasting straight baking soda, for example) In order to build up some significant amount of such ions, however, I would expect the water already has an alkaline pH that you are bubbling the CO2 through. My guess would be that you have hard water, and some reactions between the CO2 and metallic ions in your tap water (in hard water, these are often alkaline earth metals like calcium and magnesium) are creating small amounts of stuff that is throwing the taste off. The lack of fizz also indicates that the CO2 is reacting with something dissolved in the water, forming bicarbonate or carbonate salts rather than carbonic acid. This is just a guess, but I would try your system with properly distilled or deionized water to see if the flavors change.--Jayron32 15:51, 30 July 2019 (UTC)
- No, any confusion. Is really bitter, not sour. and I'm 100% sure that in the tank there is CO2 and not another gas. I don't think there is a contamination, because I use 3-liters cylinders from years, and I change the cylinders very much times. If I open and close regularly the valve, there isn't very high pressure (in the tube which connect the cylinder and the carbonator) and the water carbonate regularly, without strange tastes. The bitter (Rhubarb, coffee, chicory are bitter) taste appears only when I leave the valve open, and there is high pressure on the tube. Is possible that at high pressure the dissolution of CO2 increase and at the same time the free CO2 (who make the fizz)decrease, obtaining a less-fizzy water but with more CO2 dissolved (so more bitter)? Thank you at all--95.252.33.178 (talk) 15:15, 30 July 2019 (UTC)
Earth's Atmospheric Pressure
[edit]Where can I find a chart of Earth's atmospheric pressure over the course of the several billions of years that it has existed? Can such pressure data be reliably estimated from proxies in the same way atmospheric composition is? 182.0.213.7 (talk) 09:30, 30 July 2019 (UTC)
- I suppose gas bubbles trapped in amber could tell us the pressure at the time. You would have to radiocarbon date the recent amber, or use a similar method for older samples. Not sure if anyone has made a chart, though. Before trees made amber, I'm not sure what methods could be used. Perhaps gas bubbles in volcanic rock ? But the expansion/contraction due to temperature changes would throw that off. SinisterLefty (talk) 13:45, 30 July 2019 (UTC)
- Here's a full book, History of the Earth's Atmosphere, available via the AGU Online Library.
- Its author is also cited in several of our articles, including Paleoatmosphere.
- Here's another paper, Earth's air pressure 2.7 billion years ago constrained to less than half of modern levels.
- As you might see, the accuracy and precision with which we can confidently assess the pressure of the atmosphere is pretty low; and scientific opinions on specific values are diverse; so even if you find any chart of the air pressure over geological time scales, you should present that data in context.
- Nimur (talk) 14:09, 30 July 2019 (UTC)
- speaking of which, I am not aware of any mass balance of the atmosphere, as of today. I know it loses some gases to space, and have some gain such like volcanic gas, plus numerous exchanges with biosphere, ocean, ... but I have no idea how things add up. Does it gain matter? lose? Gem fr (talk) 14:35, 30 July 2019 (UTC)
- This field is called aeronomy. The Earth's magnetic field has a major effect on this, as it deflects the solar wind, which otherwise would eventually blow the atmosphere out into space. This appears to have largely happened on Mars, after the core solidified and the magnetic field strength decreased dramatically. When the Earth's magnetic field reverses periodically, I suspect some atmosphere is lost during the process, as the field is weak during the change (see Laschamp event). Then it must regain that atmosphere while the magnetic field is steady, or otherwise, over many reversals of the field, the atmosphere would be lost. So, since the field is steady now, logically we must be very slowly gaining atmosphere. SinisterLefty (talk) 18:17, 30 July 2019 (UTC)
- Figure 7. Earth's proposed atmospheric history concludes an argument that the giant flying creatures of the dinosaur age could only fly if the atmospheric pressure was much higher than it is now. DroneB (talk) 14:40, 30 July 2019 (UTC)
- ...and that bumblebees didn't fly (and never would). 107.15.157.44 (talk) 18:33, 30 July 2019 (UTC)
- Indeed, because of their small size and accordingly low Reynolds_number, insects are more accurately described as swimming rather than flying. Not sure the original claimant had enough knowledge of fluid mechanics, but he was right, somehow. Gem fr (talk) 19:21, 30 July 2019 (UTC)
- ...and that bumblebees didn't fly (and never would). 107.15.157.44 (talk) 18:33, 30 July 2019 (UTC)
- The oxygen percentage was higher too. If you time-traveled there and planted assorted mediterranean tree seeds in the closest thing they have to a Holocene mediterranean climate how long before a wildfire kills them? Sagittarian Milky Way (talk) 18:15, 30 July 2019 (UTC)
- Figure 7. Earth's proposed atmospheric history concludes an argument that the giant flying creatures of the dinosaur age could only fly if the atmospheric pressure was much higher than it is now. DroneB (talk) 14:40, 30 July 2019 (UTC)
- That's a good point. Plants of that era must have had some form of fire protection to survive. Bulbs far enough underground would be a way to survive, but perhaps they had something like asbestos trunks to keep from having to start over every time a fire passed through. SinisterLefty (talk) 22:49, 30 July 2019 (UTC)
- Not necessarily: plants have many kinds of fire adaptations. Fire resistance traits require resources that may be better-invested elsewhere. Some plants intentionally encourage fires that will probably kill them; they disperse their seeds when a fire happens, and the offspring can then sprout in an environment largely cleared of competition by the fire. Grasses are quite fire-tolerant and can often just re-sprout after a fire, although grasses didn't become widespread until the Cenozoic. --47.146.63.87 (talk) 00:06, 31 July 2019 (UTC)
- That's a good point. Plants of that era must have had some form of fire protection to survive. Bulbs far enough underground would be a way to survive, but perhaps they had something like asbestos trunks to keep from having to start over every time a fire passed through. SinisterLefty (talk) 22:49, 30 July 2019 (UTC)
- And then there's animals. Those which couldn't run or fly away quickly enough would have needed an underground or underwater burrow to retreat into, with enough air to last. If they could go dormant/hibernate, that would also reduce their need for air. SinisterLefty (talk) 02:25, 31 July 2019 (UTC)


