Wikipedia:Reference desk/Archives/Science/2012 December 12
| Science desk | ||
|---|---|---|
| < December 11 | << Nov | December | Jan >> | December 13 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
December 12
[edit]how did the discoverer of prussic acid / hydrogen cyanide not get like majorly killed or hurt
[edit]From hydrogen cyanide: "In 1752 the French chemist Pierre J. Macquer made the important step of showing the Prussian blue could be reduced to a salt of iron and a new acid, which could be used to reconstitute the dye. The new acid, hydrogen cyanide, first isolated from Prussian blue in pure form and characterized about 1783 by the Swedish chemist Carl Wilhelm Scheele, was eventually given the name Blausäure (literally "Blue acid") because of its derivation from Prussian blue, and in English became known popularly as Prussic acid."
Okay, so at the time the lethality wasn't noted? How do you isolate hydrogen cyanide without getting killed, if you wouldn't even have anticipated a gas? I mean, chemists regularly tasted their compounds (at the time) and it's not like they would conduct every experiment with gas masks. 137.54.1.116 (talk) 03:31, 12 December 2012 (UTC)
- Perhaps other (earlier) discoverers did taste/breathe it? The implications are left as an exercise for the reader... -- Scray (talk) 04:39, 12 December 2012 (UTC)
- Did their 18th century grad students get killed while they claimed the credit? 137.54.1.116 (talk) 04:42, 12 December 2012 (UTC)
- You know, cyanide deserves its reputation for lethality, but that doesn't mean it's impossible to overestimate it. Our article says that around 300 ppm "will kill a human within about 10 minutes". Now, 300 ppm isn't a lot, but it's not the merest trace either. If you generate 10 mL of HCN gas in a test tube, and it spreads out over a cubic meter, that's only 10 ppm, if I've done my arithmetic right (please don't count on it!). Without more details on the experiment, it's hard to know whether Macquer was in danger. --Trovatore (talk) 9:22 pm, Today (UTC−8)
- Did their 18th century grad students get killed while they claimed the credit? 137.54.1.116 (talk) 04:42, 12 December 2012 (UTC)
- Presumably they were smart enough to only taste a tiny amount of anything new, and thus received a non-fatal dosage. StuRat (talk) 05:07, 12 December 2012 (UTC)
- My understanding is that the effects occur very rapidly and are pretty obvious -- a person exposed to cyanide feels like he is suffocating. If the person recognizes that something bad is happening and gets out of there, his state won't get any worse -- he'll feel like he is suffocating for a while, and then gradually recover. So if it takes 10 minutes to get a fatal dose, a person is likely to realize that something is badly wrong long before that. Looie496 (talk) 07:34, 12 December 2012 (UTC)
- It is possible to ingest small amounts of cyanide without being affected at the time, but the build up over a period of time would kill. (I remembered reading that Napoleon Bonaparte died in such a manner, and googled "cyanide death of Napoleon". The results tell me that he died of arsenic poisoning. Does arsenic equate cyanide, metabolise to cyanide, or have I missed something here?) --TammyMoet (talk) 10:31, 12 December 2012 (UTC)
- No, cyanide and arsenic are unrelated.
- I do not think that cyanide "builds up". The body has mechanisms for detoxifying small amounts of cyanide (it has to, because cyanide is a ubiquitous molecule). So it does not make sense to me that it would "build up". What may be true is that the continued presence of cyanide causes cumulative damage that is not repaired. See for example konzo. --Trovatore (talk) 10:53, 12 December 2012 (UTC)
- I apologize if I've asked this before, but I don't recall. In The Princess Bride, the pirate builds up an immunity to the fictional poison "iocaine" over a five year period. Is it possible to build up an immunity to cyanide? ←Baseball Bugs What's up, Doc? carrots→ 11:09, 12 December 2012 (UTC)
- Our article on mithridatism specifically says that this is not possible for cyanide. It does not give a citation for this, however. So I don't really know. But the konzo experience argues against trying it, unless you really really have to. --Trovatore (talk) 11:13, 12 December 2012 (UTC)
- I would guess that it has something to do with whether the poison is an organic substance or not. ←Baseball Bugs What's up, Doc? carrots→ 05:55, 13 December 2012 (UTC)
- Our article on mithridatism specifically says that this is not possible for cyanide. It does not give a citation for this, however. So I don't really know. But the konzo experience argues against trying it, unless you really really have to. --Trovatore (talk) 11:13, 12 December 2012 (UTC)
- I apologize if I've asked this before, but I don't recall. In The Princess Bride, the pirate builds up an immunity to the fictional poison "iocaine" over a five year period. Is it possible to build up an immunity to cyanide? ←Baseball Bugs What's up, Doc? carrots→ 11:09, 12 December 2012 (UTC)
- No, you cannot build up an immunity to cyanide. Immunities mostly come from a build-up of antibodies, which cannot react to molecules which are as small as CN, or from increased metabolism by the liver. The liver cannot metabolize CN; it's its unreactivity and its affinity for hemoglobin that makes it such an effective poison. The amount that's absorbed by the red blood cells and then not given up in exchange for O2 is what kills you. μηδείς (talk) 01:41, 14 December 2012 (UTC)
- The question is not whether you can build up an "immunity" in the sense of the immune system, but about whether you can build up a tolerance. It's not absurd on its face that you would be able to do that — the body has mechanisms for detoxifying cyanide (I think mainly adding a sulfur atom to convert it to thiocyanate, which is much less toxic), and it wouldn't be surprising if that mechanism were upregulated if needed. That doesn't mean it does get upregulated, just hat it wouldn't be surprising on its face.
- Your last sentence is wrong, though it's a common misconception. Actually if you can get the cyanide to stay in your red blood cells you can tolerate a lot more of it (that's the idea behind using nitrites as a cyanide antidote — it converts some of your hemoglobin to methemoglobin, which has a higher affinity for cyanide). The way it kills you is by getting into other cells and binding to cytochrome c oxidase. Then oxygen can maybe get to your cells, but internally, they can't use it. --Trovatore (talk) 01:49, 14 December 2012 (UTC)
- Yes, you are right on the last point, I was going by memory. μηδείς (talk) 01:55, 16 December 2012 (UTC)
pKa of hydrogen
[edit]I'm trying to find the pKa of hydrogen as it is the conjugate acid of sodium hydride. However, I can't find a reliable source (searching for "pKa of hydrogen" infuriatingly reveals all sorts of irrelevant results, like the pKa of hydrogen sufide, hydrogen fluoride, basically every Bronsted acid). I want to add it to both the article for sodium hydride and this article. Can someone help me? Also, why is Google's search algorithm so terrible? 137.54.1.116 (talk) 03:45, 12 December 2012 (UTC)
- I would suggest looking in here. It's the most comprehensive list of pKa that I'm aware of. Is it right to call hydrogen gas the conjugate acid of sodium hydride rather than the hydride ion itself? It's been a while since I took chemistry, but I feel like hydride ion is the conjugate acid. In water, this ion will subsequently react with water to form hydrogen gas an a hydroxide ion. Someguy1221 (talk) 04:10, 12 December 2012 (UTC)
- Actually, I think the IP is right. The hydride ion is a Bronsted-Lowry base, being a highly good proton acceptor - hydrogen gas can be thought of as its protonated form, and thus its conjugate acid. All I know about the original question is that pKa is very very high - it's barely acidic at all.--Jasper Deng (talk) 05:22, 12 December 2012 (UTC)
- I've seen values listed around 42 (no, seriously). But I've also seen in essence "it's not really a measurable value because there's not a useful solvent in which both MH and H2 are sufficiently soluble" (need to measure the equilibrium). DMacks (talk) 19:52, 12 December 2012 (UTC)
- That seems about right. I seem to remember that butyllithium reagents have conjugate pKa values somewhere in the 50s (i.e. the pKa of the alkyl hydrogen), which would seem to be about right for hydride to fall in the 40s. I think you can derive pK values thermodynamically (i.e. ΔG=-RTlnK), so it should be technically possible to get the values via Hess's law, i.e. from the thermodynamics of a series of equations rather than via direct equilibrium measurment, that is you can get the pKa value by looking at how it reacts with other substances rather than by direct equilibrium with its conjugate. --Jayron32 05:05, 13 December 2012 (UTC)
in what types of cells, except Haploid reproduction cells, does meiosis occur?
[edit]thanks. — Preceding unsigned comment added by 109.64.147.124 (talk) 06:21, 12 December 2012 (UTC)
- None -- all other cells undergo mitosis. 24.23.196.85 (talk) 06:33, 12 December 2012 (UTC)
- Well, to be a bit pedantic, it can occur in polyploid reproductive cells too. Looie496 (talk) 07:29, 12 December 2012 (UTC)
Not quite night terrors
[edit]What is it called when someone gets frightened by some imagined or dimly-seen object in the dark (as a one-time or occasional occurrence only), even though that person is not normally afraid of the dark? It's not the same as night terrors, because night terrors are when you're half-asleep and you start seeing scary things, and it's not nyctophobia, because that is when you're always afraid of the dark. So what is it? 24.23.196.85 (talk) 06:29, 12 December 2012 (UTC)
- I would call it a standard response from a visually oriented creature to dealing with low-light conditions. Humans, like apes and most, but not all, other primates, are primarily diurnal animals which use their eyesight far more than, say, their hearing or sense of smell to interpret their environment. As such, we easily get freaked out by things in low light conditions. Your cat, for example, would never have that same response to a shadowy object. Matt Deres (talk) 15:22, 12 December 2012 (UTC)
- Wasn't hypnogogia recently mentioned here? Having suffered both phenomena, I can assure you they are quite different, and night terrors much scarier to one's boy-or girl-friend or family or spouse or children. μηδείς (talk) 00:16, 13 December 2012 (UTC)
- Thanks, Medeis and Matt! You see, I'm researching for several books all at the same time, and one of them will be about a young boy who suffers from multiple interrelated phobias -- and early on, although he is not normally afrais of the dark, he suffers from precisely this condition (a pattern of shadows in the darkened bedroom causing him to imagine things that freak him out), which gives his babysitter a good idea of precisely what he fears, and why (I don't want to give too much away, but while screaming he mentions "six-armed giants", which she guesses correctly are transmission towers). 24.23.196.85 (talk) 01:48, 13 December 2012 (UTC)
- Wasn't hypnogogia recently mentioned here? Having suffered both phenomena, I can assure you they are quite different, and night terrors much scarier to one's boy-or girl-friend or family or spouse or children. μηδείς (talk) 00:16, 13 December 2012 (UTC)
Question regarding the orbital period of the Earth around the Sun
[edit]In the article "List of gravitationally rounded objects of the solar system":
http://wiki.riteme.site/wiki/List_of_gravitationally_rounded_objects_of_the_Solar_System
the orbital period of the earth is listed in the "Planets" table as 1.000174 years. Footnote [g] indicates that these are sidereal years, but the word "years" links to the article "Julian year (astronomy)":
http://wiki.riteme.site/wiki/Julian_year_%28astronomy%29
Which is correct? In either case, how is 1.000174 derived?
Thank you for your help 50.137.168.64 (talk) 08:59, 12 December 2012 (UTC)
- I don't see any discrepancy here. The "sidereal" in the footnote means it's the sidereal period. The unit of said period is Julian years.Dncsky (talk) 10:30, 12 December 2012 (UTC)
- In other words, 1 sidereal year = 1.000174 Julian years. The sidereal year is measured - as opposed to derived. Dauto (talk) 15:01, 12 December 2012 (UTC)
- And the way it is measured is by taking precise measurement of many astronomical objects. The actual method is briefly explained at this webpage, Greenwich apparent sidereal time, provided by the United States Naval Observatory. The measurement depends on the very careful calculation of the equation of the equinoxes, and is something that astronomers spend a lot of effort to refine and work out the messy details. As our machinery gets better and more accurate, we have to compensate for more non-ideal observations - things like the non-perfect shape of the planet, and its orbit, and the change in actual and apparent positions of even very distant astronomical objects during the span of just one year, not to mention practical problems with earth-based telescopes and optics, and imprecisions in even the best clocks we know how to build, and so forth. Nimur (talk) 22:41, 12 December 2012 (UTC)
Blue sky?
[edit]Randall Munroe makes a decent point: [1]...why isn't the sky violet? I never thought about it til I saw that comic. Ks0stm (T•C•G•E) 10:17, 12 December 2012 (UTC)
- The short answer is, as the cartoon suggests, Rayleigh scattering. I don't know enough big words to give you the long answer. Evanh2008 (talk|contribs) 10:20, 12 December 2012 (UTC)
- So why isn't the sky violet?Dncsky (talk) 10:21, 12 December 2012 (UTC)
- There are dozens of very good answers here[2].Dncsky (talk) 10:22, 12 December 2012 (UTC)
- Yeah, I realized the trick in the question (violet being shorter in wavelength than blue) just after posting. My first guess is, as one of those posters suggested, that the sun emits more radiation in the form of blue light than violet. I am reading Diffuse sky radiation at the moment. Good question, BTW. Evanh2008 (talk|contribs) 10:29, 12 December 2012 (UTC)
- "The traditional way that people teach this subject is that sunlight is scattered — more so for shorter wavelengths than for longer ones," says Glenn Smith, an engineering professor at Georgia Tech. "The other half of the explanation is usually left out: how your eye perceives this spectrum." Evanh2008 (talk|contribs) 10:41, 12 December 2012 (UTC)
- Yeah, I realized the trick in the question (violet being shorter in wavelength than blue) just after posting. My first guess is, as one of those posters suggested, that the sun emits more radiation in the form of blue light than violet. I am reading Diffuse sky radiation at the moment. Good question, BTW. Evanh2008 (talk|contribs) 10:29, 12 December 2012 (UTC)

.
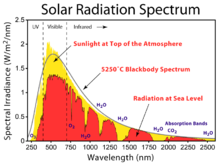
- We can eschew the complications of human visual perception by looking at the spectrum of diffuse sky light using a spectrometer (or even a simple prism). This spectrum should be compared to, e.g., the solar spectrum, to see what effect Rayleigh scattering actually has. Nimur (talk) 16:12, 12 December 2012 (UTC)
- There are no violet sensors in the eye, just three cone types all sensitive to a wide range of the visual spectrum. If you're looking at light that peaks in the green-blue-violet part of the spectrum, you're going to see what amounts to a weighted average of those spectral colors, which will be a color somewhere in the middle, not all the way at one end. That doesn't prove the sky is blue, but it does show the error in the naive argument that it ought to be violet. -- BenRG (talk) 05:00, 13 December 2012 (UTC)
- You might also look at line of purples. μηδείς (talk) 01:34, 14 December 2012 (UTC)
Burning oil at low rate using catalyst for "greenhouse".
[edit]I'm wondering about how I can heat my mother's planters to protect the plants after I've put a PVC cover over them. They're fairly small planters and I don't think they need a lot of heat. I was wondering about those catalyst hand warmers made by Zippo etc. The handwarmers only last up to 24 hours (a week or more would be better) Do you think I could use such a catalyst to burn, say, vegetable oil without a flame, at a low rate? — Preceding unsigned comment added by 78.150.23.29 (talk) 12:42, 12 December 2012 (UTC)
- I'm not sure what you mean. I don't think the chemical type would combust veggie oil, nor do I think that veggie oil would burn properly in the zippo models, which call for lighter fluid. You didn't ask for gardening advice, but depending on the location and plants, insulation and passive radiant heat are often enough to over-winter plants successfully. SemanticMantis (talk) 15:15, 12 December 2012 (UTC)
- Don't know if I'm following this right, so I'll start at the beginning. An elephant has a grater ratio of mass to surface area. Therefore, a mouse loses more body heat then an elephant. If one was to get (say) Bubble wrap and with it, construct a rude green house, then a single candle will protect all the planters placed within it - from frost. Are you wanting to protect from frost? If however, your propagating orchids, pineapples, bananas etc., please ignore this. --Aspro (talk) 19:56, 12 December 2012 (UTC)
- I think a candle might generate too much heat, too fast and damage the plants/PVC tarp, as well as needing to be frequently replaced. I'd like something that burns slowly and cleanly. — Preceding unsigned comment added by 129.215.47.59 (talk) 22:46, 12 December 2012 (UTC)
- I'm speaking from personal experience. My little Aladdin paraffin greenhouse heater threw out around half a kilowatt of heat – ruddy sight more than a candle! Yet, just a candle would keep the frost off. It gave off CO2 as well, which encourages plant growth. NOTHING burns 'cleanly' not even alcohol. They all give off pollutants... save for perhaps H2 + O2. An alternative- depending on what exactly your growing (pineapples, marijuana etc.) might befit from a just a horse-manure bed. You appear to be asking for a panacea that suits all things; and without telling us what it is your growing and on what scale.--Aspro (talk) 19:32, 13 December 2012 (UTC)
- I think a candle might generate too much heat, too fast and damage the plants/PVC tarp, as well as needing to be frequently replaced. I'd like something that burns slowly and cleanly. — Preceding unsigned comment added by 129.215.47.59 (talk) 22:46, 12 December 2012 (UTC)
- These things are for use with lighter-fluid filled hand-warmers. Lighter fluid is relatively expensive. Do you suppose they will also work with regular unleaded petrol/gasoline? I could then rig something up using a glass jar full of petrol and, if necessary, wadding. Perhaps this converter would just float on the gasoline and not need any wadding.
- Also, those catalysts are only meant to last 90 times as 12 hours per time. What do you suppose the catalyst is made of and what happens to it to limit it's life span? Could it be restored to full function after use? — Preceding unsigned comment added by 129.215.47.59 (talk) 23:04, 12 December 2012 (UTC)
- An alternative way to heat small volumes is with an incandescent light bulb. Just snake an extension cord over there, and use a plug-in socket you buy at a hardware store (you could even use an incandescent night light, if the heating needs are modest enough). This is an old technique for heating doghouses in winter, and far less likely to cause a fire. StuRat (talk) 03:07, 13 December 2012 (UTC)
- That would be good if we had an external electrical socket but since we don't it would mean compromising house security and insulation! :( — Preceding unsigned comment added by 78.150.19.212 (talk) 09:51, 13 December 2012 (UTC)
- I had the same problem. I snaked the extension cord out a 2nd story window, filled the gap along the rest of the bottom of the window with insulation, and cut a piece of wood to fit just above the window, so it can't slide up, even if a burglar were to access the window with a ladder. StuRat (talk) 04:19, 14 December 2012 (UTC)
Candle and Carbon Monoxide
[edit]Hi, a friend asked me how long would a single candle need to burn in an airtight room before it would set off a carbon monoxide detector? I'm not really sure how to find an answer, so was wondering if anyone here could be of help. For my own curiosity, what if we assume that the room has only a small crack under the door that connects it to another in door room? Note: please don't take this as a medical question, or some such, if you're trapped in an airtight room, candles would be the least of your issues:-)Thanks:-)Phoenixia1177 (talk) 13:02, 12 December 2012 (UTC)
- It would depend quite a lot on the size of the room, the size of the candle and where the detector was placed. More detail usually gets a better answer. Richard Avery (talk) 14:39, 12 December 2012 (UTC)
- There are several points to be understood here:-
- 1. A burning candle, which is essentialy a means of slowly burning a hydrocarbon wax, will produce very very little carbon monoxide, unless the oxygen level is so low you'd already be dead - most of the combustion product is water vapour and carbon dioxide, as for conbustion of any hydrocarbon.
- 2. A truely airtight room is quite difficult to achieve. A "small crack under the door" is likely to provide enough diffusion of oxygen in and carbon dioxide and water vapour out to overwhelm the output of a typical candle.
- 3. The presence of any human in the room will also consume oxygen and replace it with carbon dioxide.
- 4. There are various sorts of carbon monoxide detectors sold, and the common ones don't just react to carbon monoxide. For instance the "Cricos" detectors one of my old employers uses to detect carbon monoxide in confined spaces is routinely tested by breathing out into its' air input - by holding your breath a moment first, you can reliably trigger the alarm.
- Floda 120.145.143.148 (talk) 14:44, 12 December 2012 (UTC)
- Neither the UK Fire Service nor the US Environmental Protection Agency list candles as a source of carbon monoxide. The US National Candle Association has an interesting page on the THE SCIENCE OF CANDLES, which says that "The heat of the flame vaporizes the liquid wax (turns it into a hot gas), and starts to break down the hydrocarbons into molecules of hydrogen and carbon. These vaporized molecules are drawn up into the flame, where they react with oxygen from the air to create heat, light, water vapor (H2O) and carbon dioxide (CO2)." It quotes the 2007 Okometric Wax and Emissions Study, which says that candles "exhibit clean-burning behaviour" and "pose no discernible risk to human health or air quality". It also quotes M. Matthäi and N. Petereit: THE QUALITY CANDLE which says; "When burning candles, as in every case when burning hydrocarbons, water and carbon dioxide are produced. In addition, other combustion products can be formed in traces such as carbon monoxide, nitric oxide, organic hydrocarbons and soot." but it continues "In the case of high quality candles, which do not visibly emit soot, measurements taken have confirmed that combustion products produced are harmless" Alansplodge (talk) 14:48, 12 December 2012 (UTC)
- I agree with the above. To summarize, it won't set off the alarm, ever. (One additional reason is that the candle wax will be used up before all the oxygen gets low enough to produce carbon monoxide.) StuRat (talk) 03:11, 13 December 2012 (UTC)
- Nice answers, all of you. – b_jonas 09:30, 13 December 2012 (UTC)
Critical Illness cover
[edit]Questionable whether this is a science desk question, but I regarded it as so. I work for an insurance company and recently got critical illness insurance for a little over 1 GBP a month, with a payout of £25,000 on diagnosis of a specified list of critical illnesses (the usual you would expect - cancer, heart attack etc). My question is, how is this so cheap? This would imply that the amount of people my age (25) getting a critical illness is 1/25,000 of the population every month? This seems very low but cannot find any statistics for people this young, only 65+ (Where 1GBP a month would be a bargain - so presumably is much more expensive for the over 65s). Thanks for any help! 80.254.147.164 (talk) 13:20, 12 December 2012 (UTC)
- A combination of factors; which applies specifically to you depends on your circumstances: 1) you've got an employee discount 2) there's a deductable 3) 25K is the max payout, one they only pay for the worst cases (compare to the disfigurement and body-part loss numbers for cheap travel insurance - losing a finger only gets you £400 and a leg only £3000) 4) check their delineation between critical and chronic illness - if you get Hepatitis-B or Malaria (which are going to affect you for the rest of your life), the probably won't pay for anything beyond the initial episode 5) they think they can recover some of their costs from other insurers (e.g. your travel insurance, or the liability insurance of involved parties) 6)they only insure you for out-of-pocket expenses; in the UK the NHS pays up the lion's share of the healthcare costs 7) if the insurance covers you for lost wages, they're relying on people your age being fairly low earners, and on employers having to pay statutory sick pay. For someone your age, who doesn't already have a history of chronic illness, that 1/25000 is probably about right. -- Finlay McWalterჷTalk 13:47, 12 December 2012 (UTC)
- Read the limitations and exclusions. Probably non-terminal cancer is not covered, and due to advances in medicine most cancers aren't terminal. Heart failure might kill you right away. HIV might be excluded, unless accidentally infected, and this is rare. And insuring 25 year olds for Alzheimer, heart valve replacement and Parkinson is a joke. If you are part of the NHS, I don't know if you need this kind of lump sum insurance, although you could cover the basics (health, disability, death, property) with some extra insurance. OsmanRF34 (talk) 22:18, 12 December 2012 (UTC)
- A quick scan says that in the UK the rate of cancer diagnoses per month for 25 year old males is about 1 in 500,000; for heart disease around 1 in 100,000. Those are probably the two most common critical illnesses in this age group, so there is plenty of room for the insurance to be profitable. Note that we have an article on critical illness insurance. Looie496 (talk) 01:41, 13 December 2012 (UTC)
word for a type of visual disturbance
[edit]I am curious if there is a term for a type of visual disturbance where the items one is looking at (letters on a page, for example) seem to swim about a bit, maintaing their relative position, and being perfectly legible, but seeming to shift a bit side to side and up and down like dead leaves floating on the surface of a pool. Searches have led me to blurred vision and floaters, neither of which is at all what I mean. Thanks. μηδείς (talk) 18:04, 12 December 2012 (UTC)
- Sounds a bit like one form of dyslexia. According to our article: "... dyslexic readers ... move letters around when reading – this only occurs in a very small population of dyslexic readers". StuRat (talk) 18:20, 12 December 2012 (UTC)
- You say that they "seem" to exhibit the visual quality that you go on to describe. What is instrumental in bringing about the "visual disturbance" that you describe? Can you link to an example of this? Bus stop (talk) 18:28, 12 December 2012 (UTC)
- Sorry, I can't help provide a name, but I will at least clear up that it is in no way similar to dyslexia. What Medeis is describing (and which I've also been curious about) is the visual trick where letters or some other pattern is printed in bright, saturated colours against a background of a dissimilar bright, saturated colour. I most often notice it with bright red printing against a bright blue background, such as what might be on the cover of a children's colouring book. As you move your head, the letters or patters seem to "shudder" slightly, as if they're not attached to the page. Matt Deres (talk) 18:44, 12 December 2012 (UTC)
- After some casting about on Wikipedia, I'm thinking it's a form of "active" afterimage, where the high contrast and sharp borders allow you to experience the afterimage almost directly on top of the original image. Matt Deres (talk) 18:53, 12 December 2012 (UTC)
- Hallucination suggests this could be considered a type of pseudohallucination, since you are are that your perceptions are "not real". I suspect you are looking for a more specific term, but I'm not sure there would be a rigorous scientific classification of something so subjective. If you are ok with folksonomy, you'd probably get some good answers at the fora of erowid. If you are interested in WP:OR and guesses, then I also think I have perceived what you and Matt are talking about, for me I assumed it had something to do with eye fatigue, different focal points for each eye, and maybe eye wetness. SemanticMantis (talk) 18:47, 12 December 2012 (UTC)
- I did find Scotopic sensitivity syndrome, which is a controversial "syndrome" and not apparently recognized widely, but which mentions "Text that appears to move (rise, fall, swirl, shake, etc.)" as a symptom. I have no idea if that particular symptom is unique to the syndrome or not. There may be others at Category:Visual disturbances and blindness. --Jayron32 18:52, 12 December 2012 (UTC)
- Complementary colors are said to "clash". A Google search for complementary colors clash might reveal some relevant links. For instance: "A pair of complementary colors printed side by side can sometimes cause visual vibration (clash) making them a less than desirable combination."[3] Bus stop (talk) 18:54, 12 December 2012 (UTC)
- Like this ? I was going to suggest that it might be caused by sneezing on the screen or reading in the rain which would have been foolish. Luckily, I didn't. Sean.hoyland - talk 19:00, 12 December 2012 (UTC)
- (ec) It hearkens back to my once taking ololiuqui in the 1980's and the visual effects. The initial experience was quite extreme, but for the following month or so I had "flashbacks" where items in my field of vision, especially letters on a page, dots in paint on a wall, or the tiles on the floor would seem gently to shift side to side, very reminiscent of, say, looking down from above on boats crowded together on the surface of a body of water, not exchanging places or being indistinct or confusing blurry, and not at all illegible in the case of letters, just not maintaing equal distance. I have seen similar effects on cartoons or depictions of people tripping, although that's usually a lot more disturbed than what I am describing. Having just picked up Oliver Sacks's latest, hallucinations, brought the idea back to me and made me want took it up on line. Unfortunately searches for things like "swimming letters" get hits on letters of permission or commendation for swimming events, and most of the rest I am getting has to do with floaters in the eye cavity, which is not what I am looking for. μηδείς (talk) 19:28, 12 December 2012 (UTC)
- Some of this effect is due to dispersion in the eye, so that different colours require a different focus from the lens. Where they abut each other it is hard to focus on both edges at the same time. Graeme Bartlett (talk) 20:20, 12 December 2012 (UTC)
Okay, I have just read through the very interesting responses above in full. None has quite hit it on the head. It's definitely not dyslexia, which I have never had under any circumstances. It's not an after image, since there was only one clear image--simply that items which I knew were fixed seemed to be swaying slowly relative to each other, but my field of vision was clear and I could read (when I was reading) without a problem. Bus Stops' link to complementary colors leads to a different phenomenon with which I am familiar--but the author in that case seems to be using the word vibrate metaphorically. Sean's image is actually very helpful, since, if you ignore the color differences and assume they instead indicate a slow swaying motion of black on white text (and word-by word, rather than letter by letter) you will get a good idea of what I am talking about. The scotopic sensitivity syndrome Jayron mentions matches in only that one aspect; the "text that appears to move (rise, fall, swirl, shake, etc.)" but is otherwise unconnected.
SemanticMantis is correct that this would have to be described colloquially as a pseudohallucination, since it occurred in my experience when I ate morning glory seeds in the 80's and tripped very strongly while aware I was hallucinating, along with "flashbacks" which of the phenomenon I am trying to name, and yet again around the turn of the century when I was hospitalized and had to have several major abdominal surgeries. In the later circumstance I went for three days or so without sleeping, and had the same visualizations; that words on text or spots on paint or abstract patterns on the wall were "crawling" or rows of tiles on the bathroom floor were not quite keeping parallel. (Just now I am reminded of Star Trek's "distorted space".) They went away when they made sure I got a good night's sleep.
I think if we had a list of visual hallucinations or list of visual disturbances I might possibly come acrost a term that matches what I am looking for. If anyone's got any suggestions as to such categories here or on other sites it would be very helpful. μηδείς (talk) 21:35, 12 December 2012 (UTC)
PS, Graeme, can you give an example of what you're talking about? I don't think it's the exact phenomenon, but I am curious. μηδείς (talk) 21:54, 12 December 2012 (UTC)
- This seems most likely to be nystagmus. The morning glory seed effect is definitely due to nystagmus -- that mechanism is well understood. (Ironically, this question could be interpreted by a pedantic person as a request for medical advice, since various neurological issues could cause this to happen.) Looie496 (talk) 22:35, 12 December 2012 (UTC)
- No, I am not seeking medical advice, unless there is some way to prevent symptoms that last occurred well over a decade ago, and the memory of which one does not need to be rid of. I simply want to read the literature on the phenomenon. Nystagmus sounds relevant and interesting, but the article is about a diagnosis or a physical act, not an experience as such. I am more looking for what the psychological experience might be called, than rather than what its cause might be. 00:06, 13 December 2012 (UTC)
- Our article does not explain it very well. What happens physiologically in nystagmus is that the eyes move slightly when you try to keep them pointed at something; the experience is that the thing you are pointing them toward seems to move. Looie496 (talk) 00:23, 13 December 2012 (UTC)
- No, nystagmus does not result in the type of perception Medeis is describing, but rather in vision with inconsistent focus. You have to bear in mind that the eye is constantly darting back and forth as is, quite independent of our conscious efforts, and yet we still form visual constructs with stable relative positions; our brain forms the composite well after the involvement of the eye. Point in fact, we don't see anything but the tinniest fraction of what we think we're looking at; our visual cognition centers fill in the gaps using context such shading, surrounding colors and patterns, presumed orientation of light sources and any number of other assumptions that evolution adapted our brains to predict. The fact that we rely so heavily on this post-sensory processing is why we are also susceptible to a large variety of optical illusions. Nystagmus causes movements of the eye which are not consistent with the brain's necessary pattern of assimilation of those little chunks from which it abstracts the image as whole, post-optic nerve; it should cause an intermittently blurry field of vision with a difficult to focus on specific objects, especially at a distance --and to the best of my knowledge, that's what it does-- rather than a static image with certain elements that seem to float above it but in relation to one-another as Medeis describes. This would have to be the result of a neurological component, which is consistent with what we know of the context in which he experienced this hallucinatory phenomena. However, also note that seeing as the substance in question can influence a wide variety of modules for visual perception, with inconsistent results, it could be very hard to nail down exactly what kind of visual aberration/sensation was being experienced, especially from a thirty-year-old self-report from while under the influence! No offense to Medeis or his intriguing inquiry intended. Snow (talk) 10:42, 13 December 2012 (UTC)
- Could it be related to an ocular migraine? The wp article isn't too good as it doesn't make it clear that they are often painless, but this explains it better. I know the symptoms are different, but perhaps it could be an atypical form. Richerman (talk) 00:28, 13 December 2012 (UTC)
- On the focussing story here are the references: K Mitton claims that blue cannot be focussed.[4] Timothy claims that the fovea centralis has no blue cones, and so cannot focus blue or violet.[5] Milton Katz describes the difference in focusing colours on different parts of the retina.[6] Graeme Bartlett (talk) 10:09, 13 December 2012 (UTC)
- I think an earlier question of mine was about this same phenomenon, which I tend to notice mostly in regard to red hues. Wnt (talk) 17:24, 13 December 2012 (UTC)
Well, no, this still really has nothing to do with color, since I can recalling it happening with the text of a book, brown slashes on cream wallpaper, cream dots on blue wall paint, and bathroom tiles of various sorts. And there was no halo effect or anything one gets with optical illusions dealing with colors. To try one last analogy, imagine a kidnapper's note from a movie, or white scrabble tiles with black lettering floating on a white surface like a pool filled with ping pong balls. Imagine that the words or letters (this seemed to be more by words and rows of words than by letter with text) slowly swaying back and forth in relation to each other, but otherwise being perfectly in focus and with no other distortions but the movement.
I have also read Oliver Sacks's Migraine, and neither it nor the illustrations it holds match the phenomenon I want to put a name to. But I do think the effect is probably neuro-logical/-chemical, rather than mechanical or physiological. At this point I would simply be happy to have a list of visual distortions or the like as such. Thanks. μηδείς (talk) 23:00, 13 December 2012 (UTC)
- Medeis, one question that might help to narrow things down a bit: did any of these objects ever slide completely from your field of vision or seem like they were about to if you didn't follow their "movement", only for them to pop suddenly back to their original location and begin sliding again? Snow (talk) 00:20, 14 December 2012 (UTC)
- The only time where I would have been able to make such a judgment was reading text, since in the other cases the speckled paint or wallpaper or bathroom tiles pretty much filled my field of vision. In the case of text, no, nothing was sliding off the page, or needed to be chased. Another analogy might be a curtain or a decorative net of the type one might find at a seaside resort with shells and dried sea-creatures hanging from them, swaying in the breeze. The distance between the items might shift back and forth, but they would maintain their relative arrangement and nothing would disappear. In fact, had I not been gazing at these items I might not even have noticed there movement. Perhaps there is a vocabulary of "tripping"? I found when I was having the same pseudohallucination in the hospital the psychologist they had me talk to had trouble understanding what I was describing, and even had orderlies called when i said I was hallucinating. It took me a few minutes to convince her I wasn't having a break with reality, just seeing motion I knew wasn't real. μηδείς (talk) 01:33, 14 December 2012 (UTC)
- I see, so a general "warping" of the visual field, but restricted to the spaces between distinct objects and not the objects themselves? Well, collectively disorders effecting the perception of size and shape are known as metamorphopsias, so you might start there. However, these are usually the result of brain trauma/disease and usually involve distortion of the ability to accurately perceive relative size and shape of all or part of any object, rather than your situation where the objects (or rather letters or other items on a flat plane) themselves remain unaltered and the distances between them waver. This implies that your object constancy (or the equivalent modality for letters as we actually utilize highly specialized pathways specifically for the recognition of letters and other abstract symbols). So now that I think about it, the phenomena shares more in common with certain types of palinopsia (which actually includes a broader range of disorders involving the placement of objects in the visual field than our article suggests) and other conditions involving the parietal lobe. In any event, I'll have to check some more resources; I'm sure this one must be described somewhere in the literature of visual disorders, though I'm not sure it has an established definition or pathophysiology. Snow (talk) 03:05, 14 December 2012 (UTC)
- Yes, I am not about to trip again, intentionally, ever. But yes, the words in the text retained their size, but the spacing between and above and below them seemed to fluctuate. Object constancy is a correct term from my study of perception. I'll have to look into palinopsia.
- I have reread palinopsia. There was no hemianopsia, no trails, no snow. I'll read the other articles that are linked as see also, but there were no field of vision losses--again, only and solely an alteration in the distance between perceived "entities". μηδείς (talk) 03:32, 14 December 2012 (UTC)
- Yeah, for some reason our article on palinopsia only addresses a subset of these conditions (the ones relating specifically to afterimage) and does not reference the other varieties of distortion to visual perseveration. What I expect would be happening here is that the substance in question was playing havoc in your occipital lobe; one of the many visual tasks that take place in this region is the affixation of "retinal coordinates" to elements of incoming visual stimuli. If this process were to be disrupted, then certain stimuli detected could lose its appropriate place in the mental construct that is your visual field. However, because this effect would not be acting equally and consistently upon the neurons making up this pathway, certain objects were being represented in their "true" positions, and others outside them, with the rest of your visual cognition suite running to play catch-up to put the distorted image together from context. The robust and largely independent centers for recognizing writing kept the letters as distinct recognized and constant "objects" but the disruption was still enough to keep their actual stable positions from being faithfully represented in your composite perception. That's a fair bit of speculation, but I'd bet it's something along those lines. I still have yet to find a specific reference to this exact phenomena, but there are any number of optical illusions which exploit similar mechanisms as those described above to create a warping effect. Snow (talk) 04:56, 14 December 2012 (UTC)
- Yes, I fully agree with your analysis. I actually took Psychopharmacology 301 (or whatever it was) before I decided to try LSD by eating morning glory seeds. I am just vexed at this point we don't have an article on the symptom itself. μηδείς (talk) 01:52, 16 December 2012 (UTC)
- I once saw a Rolling Stones video (I don't remember the song; 1996?) with an effect that I sometimes had with LSD. Imagine watching a movie projected on a lumpy white wall. If your eye is near the projector, it looks okay; but when you move ... you get this effect. —Tamfang (talk) 07:50, 26 June 2013 (UTC)
wrist-mounted mini rocket launcher?
[edit]As sometimes seen in video games, anime, etc.. A weapon worn on the outer wrist that can fire out a rapid-fire volley of small, but high-explosive rockets at a given target.
Does (or did?) any such weapon exist in real life, even if it was only a prototype? I suspect that in real life, the high likelihood of setting one's own arm on fire would probably make it a largely unworkable concept... --Kurt Shaped Box (talk) 22:41, 12 December 2012 (UTC)
- Well, "only a prototype" virtually ensures that the answer is "yes". Youtube shows people making wrist-mounted launchers. They're frequently pneumatic and fire darts and other non-lethals, but it's no stretch to replace "pneumatic" with "rocket" (model rocket engines are of reasonable scale) and "Nerf" with "C4". That said, I feel reasonably confident saying that no such device has yet been seriously considered for fielding by any modern military. In addition to the heat problem you note, rockets of that scale confer neither an accuracy nor a firepower advantage over a conventional rifle, grenade launcher, or likely even a hand-thrown grenade. — Lomn 23:04, 12 December 2012 (UTC)
- On the plus side, the efflux from the rocket motor would remove all those unsightly hairs on your arm, although it might remove your skin in the process. Alansplodge (talk) 01:01, 13 December 2012 (UTC)
- Wouldn't such a rocket launcher be useful as a concealable weapon? 24.23.196.85 (talk) 01:53, 13 December 2012 (UTC)
- You may be interested in Gyrojet, which was a family of firearms including pistols that fired very small rockets. Why isn't every army using these, you ask? Because they suck nuts. Army testers reported that the guns jammed easily, rockets failed to ignite at least 1% of the time, and they had poor accuracy. In all fairness, they were designed in the 1960s, so perhaps modern technology could produce a more reliable minirocket, but this experiment probably killed the Army's enthusiasm for the idea for a long time. On the plus side, the guns are very light and have low recoil. Someguy1221 (talk) 01:43, 13 December 2012 (UTC)
- Now let me make some suggestions to get such a device to work:
- 1) Launch it several meters by means other than the rocket, such as explosives, just like in a bullet, then ignite, once it is far enough from the person to avoid burning them.
- 2) Add a guidance system, such as a heat seeking missile, so you can actually hit the target. StuRat (talk) 03:17, 13 December 2012 (UTC)
- It's not necessary you chime in on every topic you know some tangential amount about stu. Shadowjams (talk) 04:00, 13 December 2012 (UTC)
- Good one, 182 IP! :-D 24.23.196.85 (talk) 06:38, 13 December 2012 (UTC)
- I can't resist adding my oblique, hyperbolic take on things and/or adding a secant opinion, even it makes me seem eccentric, at times. StuRat (talk) 07:51, 13 December 2012 (UTC)
- Stu's in here? Then I'll drop my $.02 here too...
- Re 1) "Several" meters is quite a low requirement. Pneumatics excel here (up to ~300m); micro-rockets are over-complicated. And at low speed, rockets transfer most of their energy into the reaction mass rather than the payload. Pneumatics and slowly burning propellants are widely used in 20mm and 40mm grenade launchers, and their arc is more predictable because most of the trajectory is in free fall.
- Re 2) That would be quite expensive and, while homing in on vehicles quite well, next to useless against humans. Esp. with the difficulty at aiming, you don't want to waste any shots.
- On top of that, you can't aim it that well (which becomes a major point if you change the range requirement to several 100s of meters). I've tried with paintball guns ducttaped to my arms. Trust me, it sucks. Rifles and shoulder-launched rockets are easier to aim than anything attached to your forearm.
- Unless you can lick your own elbow. - ¡Ouch! (hurt me / more pain) 09:55, 13 December 2012 (UTC)
- 1) You seem to have misunderstood. The several meters is just to get them clear of the soldier, before the rockets fire and take them the rest of the way.
- 2) The price of miniature electronics is always going down, so it may become more of an option soon. Heat seeking missiles could also be used as an anti-personnel weapons, provided the background temperature isn't near body temperature. They'd also be more useful at the start of a battle, since later many items might be on fire. They could be particularly useful for hitting targets you can't see, like by going over a wall first. StuRat (talk) 18:58, 13 December 2012 (UTC)
- 1) you're right Stu, I mixed that up. When I read "ignite", I thought of igniting an incendiary warhead, not the rocket motor. Somehow one of those USB desktop "rocket" launchers came to mind. Those gifts from coworkers which you try once and never again. :( You were talking about a "multi-stage" design (chemical propellant followed by rocket), which is actually quite clever. First give the rocket some speed, then ignite the rocket.
- However, reinforcing the launcher would make the rocket redundant, and allow for heavier payloads, for more of them, or for a more compact launcher design.
- 2) Let's say the price of the seeker head is that of a decent digital camera plus some coolant (to minimize noise), plus some 200% because it isn't in mass-production yet. (This may go down in future but given gyrojet prices, it won't.) Now if you use a rocket, you need more coolant. If you don't, you can still launch it in a free-fall arc, say over a wall or into a window - grenade launchers are accurate enough to do that qwithout seekers. Now you can use a seeker or just use a "big" frag warhead. I'd use the frag, for the seeker cannot tell hostage from terrorist either (so both are useless then), and if there are no good guys around, I want to frag as many of the bad guys as possible with the first shot.
- One more thing, body temperature (310K) is "near" room temperature (294K). At night, that difference might be sufficient, but when the sun is shining, the average car roof has a higher IR signature than humans. (IR looks like visible light then, except that really hot objects are glowing.)
- Electronics do get cheaper, but at the same time they get worse. YOu get phones which can't even make a good call, and rockets which wouldn't even clear the launch tube. No, really, you won't get a good enough AI into the space of, say, a 12-gauge shell or a 20mm grenade by 2030.
- Another thought, a single loud *POP* would not give your exact location away like a volley of rockets would. A Gauss gun sounds like the perfect launcher: precise, powerful, and no *POP* at all. - ¡Ouch! (hurt me / more pain) 08:42, 14 December 2012 (UTC)
- 2) The price of miniature electronics is always going down, so it may become more of an option soon. Heat seeking missiles could also be used as an anti-personnel weapons, provided the background temperature isn't near body temperature. They'd also be more useful at the start of a battle, since later many items might be on fire. They could be particularly useful for hitting targets you can't see, like by going over a wall first. StuRat (talk) 18:58, 13 December 2012 (UTC)
- I don't see the comparison with digital cameras. You don't need any buttons, display, internal memory, memory cards, attractive case, shutter, etc. Much lower resolution would be fine (maybe 100×100 pixels ?), and you don't need to focus. A blurry, low-res image can still tell you which pixel is brightest. Such a weapon would also be useful against an enemy hiding in a trench (the guidance system would aim it into the trench from above). StuRat (talk) 18:57, 14 December 2012 (UTC)
- This is a concept so impractical that it didn't even work out for Bobba Fett in a fictional universe that rewards ridiculous weapon choices. It doesn't fair any better in reality, I'm afraid. Now, strapping them to penguins on the other hand... Snow (talk) 11:55, 13 December 2012 (UTC)
- There was a quote by a 19th century British general about the Congreve rocket system which went something like; "If military rockets had been invented before guns, we would all be thinking what a wonderful invention the gun was". Alansplodge (talk) 15:25, 14 December 2012 (UTC)
- Hmmmm, but rockets have the ability to continually alter their trajectory in flight. Very intelligent rockets should be able to pursue a fleeing target around corners, compensate for a fast-moving target trying to evade, brake to deliver a sedative injection without injuring the target, etc. Maybe the balance will shift in their favor? Wnt (talk) 06:07, 16 December 2012 (UTC)
