Wikipedia:Reference desk/Archives/Science/2011 September 4
| Science desk | ||
|---|---|---|
| < September 3 | << Aug | September | Oct >> | September 5 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
September 4
[edit]Hurricane and tornado
[edit]Is it possible for a tornado to exist in the eye of a hurricane?Conrad Bunger (talk) 00:46, 4 September 2011 (UTC)
- No. The conditions in the eye of a hurricane are nothing like the conditions under which tornados occur. --Jayron32 01:30, 4 September 2011 (UTC)
- Our article Huricane#Effects states: Tornadoes can also be spawned as a result of eyewall mesovortices, which persist until landfall. -- 110.49.234.147 (talk) 02:35, 4 September 2011 (UTC)
- Yes, but the eyewall is not the eye. --Jayron32 02:48, 4 September 2011 (UTC)
- Granted, but do you know what direction tornadoes spawned by the eyewall necessarily travel, or does it just seem counterintuitive that they could travel through some portion of the eye?
Is that a polite enough way of saying "citation needed"?Actually, I'm not looking for a citation, just seeking to understand the source of your certainty. -- 110.49.233.47 (talk) 06:04, 4 September 2011 (UTC)
- Granted, but do you know what direction tornadoes spawned by the eyewall necessarily travel, or does it just seem counterintuitive that they could travel through some portion of the eye?
- It seems counter-intuitive to me. A tornado can't exist in good weather, which is what you have in the eye. StuRat (talk) 06:12, 4 September 2011 (UTC)

- Mesovortices have actually been observed to cross the eyes of hurricanes. And they've been documented to cause tornadoes in the interior edges of hurricane eyewalls. Nevertheless, mesovortices (which in turn cause tornadoes, particularly the mesocyclones in supercells) only form in the hurricane eyewalls, where wind speed and wind shear is greatest. They require rising air to form, and hurricane eyes have zero convection and only gently sinking air. While they can survive crossing the eye, they will be less likely to form tornadoes the farther they are from the eyewalls and probably not at all in the center.
- In a similar but far smaller scale, small vortices can also form within larger vortices, resulting in multiple vortex tornadoes (not to be confused with satellite tornadoes and tornado families). See this illustration of one.
- Also superficially similar but not really related, heh: when winds are strong enough, the eye of a hurricane can contract, resulting in a pinhole eye. It can then trigger eyewall replacement cycles, in which a new eyewall forms outside of the original smaller eyewall, creating a sort of eye within an eye. The outer eyewall will eventually contract, 'killing' the smaller eyewall and leaving a larger eye. However, these are not tornadoes.-- Obsidi♠n Soul 13:08, 4 September 2011 (UTC)
- Thankyou Obsidian.Conrad Bunger (talk) 08:59, 5 September 2011 (UTC)
- Finding a tornado which has drifted into the area of the eye is only possible in the "anything is possible" sort of way. It would not be an expected, or predictable, thing to happen. --Jayron32 12:54, 4 September 2011 (UTC)
- I'm truly sorry, but honestly you are annoying. I realize you are trying to help, but you aren't.Conrad Bunger (talk) 08:59, 5 September 2011 (UTC)
- Also superficially similar but not really related, heh: when winds are strong enough, the eye of a hurricane can contract, resulting in a pinhole eye. It can then trigger eyewall replacement cycles, in which a new eyewall forms outside of the original smaller eyewall, creating a sort of eye within an eye. The outer eyewall will eventually contract, 'killing' the smaller eyewall and leaving a larger eye. However, these are not tornadoes.-- Obsidi♠n Soul 13:08, 4 September 2011 (UTC)
African Americans and eGFR
[edit]The CMP I just got back makes a distinction between the value of my eGFR based on whether I'm African American or not. The article on eGFR states that is because "It is known that African Americans...have a higher amount of muscle mass than Caucasians; hence, African Americans will have a higher serum creatinine level at any level of creatinine clearance." That sounds like a very controversial claim (i.e. the first one, about muscle mass) – is this generally accepted within scientific community? Is it openly applied in any other medical contexts besides GFR?
--Alfonse Stompanato (talk) 02:19, 4 September 2011 (UTC)
- See Sickle-cell disease --DeeperQA (talk) 02:35, 4 September 2011 (UTC)
- How is that relevant? --Mr.98 (talk) 14:39, 4 September 2011 (UTC)
- As a side question, the use of the term African American in Renal function#Creatinine-based approximations of GFR seems rather U.S. centric. Is this data known only for Americans, or is it know for Europeans of African descent and Africans as well? -- 110.49.234.147 (talk) 03:01, 4 September 2011 (UTC)
- Social, political and racial preferences are often used to avoid pejorative risk. --DeeperQA (talk) 04:35, 4 September 2011 (UTC)
- ??? --Mr.98 (talk) 14:39, 4 September 2011 (UTC)
- Social, political and racial preferences are often used to avoid pejorative risk. --DeeperQA (talk) 04:35, 4 September 2011 (UTC)
- I work with eGFR with many of the papers I work on. It is very rare that "African American" is used. The term is nearly always "Black". Medicine doesn't suddenly change how it works when you leave the United States, so medical papers are not written for whatever the political whim of the day may be. However, when a paper is being sent to a political group, such as DHEC, all references to "Black" are replaced with "African American" to the point of nonsense (ie: African Americans in Nigeria have higher rate of progression to hypertension than whites.) -- kainaw™ 22:49, 4 September 2011 (UTC)
- It's really quite remarkable — I hadn't heard of such a thing, but Googling around, that's definitely the explanation given by numerous "reputable" sites (e.g. the National Kidney Foundation and the American Kidney Fund and the NIH). It must be based on something; it would be a curious thing to trace this backwards in the literature to see from whence it derives, what it is based on, etc. Looking around Google Scholar a bit, I found this article, which interestingly tries to see if the differences in kidney function correlate with actual genetics, or from social and environmental factors, and concludes that the latter are at fault. Which would imply that it is not something that would necessarily apply in other nations. Fascinating stuff! --Mr.98 (talk) 14:39, 4 September 2011 (UTC)
- I think this is being read incorrectly. The assumption is that blacks with renal disease have higher muscle mass than other races. The measurable difference is clear: age. Blacks have renal disease at a significantly younger age. It is assumed that at a younger age, people in general have more muscle mass. Therefore, blacks with renal disease have more muscle mass because they are younger. That assumption is disputed, but it isn't very important. The reason why there is a difference in serum creatinine clearance isn't as important as trying to prevent renal disease in the first place. -- kainaw™ 22:56, 4 September 2011 (UTC)
- This seems like an important cultural question. I've occasionally seen expansive claims for race-specific medicine like this. It seems like we've all learned that a claim of genetic racial differences in intelligence or criminal behavior is almost surely bogus - based on small study size or environmental circumstances, socioeconomics, ability to play ball games etc. So why indulge such hypotheses when dealing with other measurements, without extraordinary proof? After all, if muscle mass is the reason (for example) then surely it isn't that hard to estimate with a scale and a tape measure. Wnt (talk) 18:35, 5 September 2011 (UTC)
- Perhaps I wasn't clear. The claim is that younger people have more muscle mass. Because blacks with renal disease are significantly younger than other races, they have more muscle mass. It isn't a comparison of race. It is a comparison of age. Muscle mass is not considered a reason that blacks have renal disease at a significantly younger age. -- kainaw™ 23:59, 5 September 2011 (UTC)
definition of space
[edit]Space is defined traditional as the place which does not allow too masses to occupy at the same time. Since energy and mass are interchangeable does that mean space is a place which does not allow mass and energy to occupy at the same time? --DeeperQA (talk) 02:25, 4 September 2011 (UTC)
- On a quantum mechanical level, space doesn't really have that kind of exclusivity that you're hypothesizing. For example, an atom can have two different atomic orbitals, both of which are occupied by electrons, such that for significant bits of space at a specified time, the wavefunction is nonzero for both orbitals. One less technical way of describing that situation would be to say that more than one electron is occupying the same bit of space at the same time. Red Act (talk) 03:02, 4 September 2011 (UTC)
- Yes, but only if they have opposite spin. Opposite spin allows their wavefunction to be orthogonal so that there is no interference. Orthogonal waves may pass through the same point in space at the same time without any effect on each other. and there are particles which can occupy the same place at the same time, Bosons for example. I have no idea where the defining characteristic of space is the place where two masses cannot coexist at the same time. That is neither a) what space is nor b) true. --Jayron32 03:10, 4 September 2011 (UTC)
- It doesn't have to be spin that the electrons differ by in order for the Pauli exclusion principle to not be a restriction. A difference of any of the electrons' other three quantum numbers (i.e. n, l and ml) will also allow for electrons with spatially overlapping wavefunctions. Red Act (talk) 04:29, 4 September 2011 (UTC)
- Entirely true, but changes to the three other quantum numbers for an electron create situations where the wavefunctions only partially overlap. The whole point is moot, however, since the idea of an "electron" occupying a "location in space" is a misguided understanding of what an electron is anyways. --Jayron32 04:36, 4 September 2011 (UTC)
- I agree completely. Red Act (talk) 05:33, 4 September 2011 (UTC)
- Entirely true, but changes to the three other quantum numbers for an electron create situations where the wavefunctions only partially overlap. The whole point is moot, however, since the idea of an "electron" occupying a "location in space" is a misguided understanding of what an electron is anyways. --Jayron32 04:36, 4 September 2011 (UTC)
- It doesn't have to be spin that the electrons differ by in order for the Pauli exclusion principle to not be a restriction. A difference of any of the electrons' other three quantum numbers (i.e. n, l and ml) will also allow for electrons with spatially overlapping wavefunctions. Red Act (talk) 04:29, 4 September 2011 (UTC)
- Energy is either an inherent property of photons or mass, it doesn't make sense to talk of energy as an object. It's like talking about length, or speed of an object. It would be like saying: this object's velocity is located over there - it doesn't make sense. All you can say is that the mass has this energy or the photon has that energy. Plasmic Physics (talk) 03:19, 4 September 2011 (UTC)
- Why are you singling out photons in you explanation? All particles have energy associated with them. Dauto (talk) 04:29, 4 September 2011 (UTC)
- Yes, but only if they have opposite spin. Opposite spin allows their wavefunction to be orthogonal so that there is no interference. Orthogonal waves may pass through the same point in space at the same time without any effect on each other. and there are particles which can occupy the same place at the same time, Bosons for example. I have no idea where the defining characteristic of space is the place where two masses cannot coexist at the same time. That is neither a) what space is nor b) true. --Jayron32 03:10, 4 September 2011 (UTC)
- @OP Your definition of space is very peculiar. I don't think I ever saw space defined that way. You're confusing space with property of matter (as described in physics books at elementary level) that two different bits of matter cannot occupy the same spot in space at the same time. Now, that's not a very good definition of matter either, but at least a case can be made that that's a traditional way to define matter. Space is traditionally defined simply as a set of coordinates. Dauto (talk) 04:29, 4 September 2011 (UTC)
- Like the Thanator of Pandora I pursue comprehension by atacking from any possible side of the tree. --DeeperQA (talk) 04:51, 4 September 2011 (UTC)
- That would be fine except that you may be attacking the wrong tree. Dauto (talk) 07:02, 4 September 2011 (UTC)
- I saw a particle diagram not long ago that showed where the Higgs Boson fit in. The interesting thing is that each particle was listed according to its mass to energy ratio with the Higgs expected to have the most mast but with size of all the particles being about the same as an electron which itself was listed as a particle external to the nucleus. This is the energy I am referring to; the mass to energy ratio as distinguished from the energy of particle motion. --DeeperQA (talk) 12:25, 4 September 2011 (UTC)
- Either that diagram was wrong or you have misinterpreted it. E=mc² tells us that the ratio of mass to energy takes the same value for all particles in all circumstances. Gandalf61 (talk) 12:42, 4 September 2011 (UTC)
- I saw a particle diagram not long ago that showed where the Higgs Boson fit in. The interesting thing is that each particle was listed according to its mass to energy ratio with the Higgs expected to have the most mast but with size of all the particles being about the same as an electron which itself was listed as a particle external to the nucleus. This is the energy I am referring to; the mass to energy ratio as distinguished from the energy of particle motion. --DeeperQA (talk) 12:25, 4 September 2011 (UTC)
- That would be fine except that you may be attacking the wrong tree. Dauto (talk) 07:02, 4 September 2011 (UTC)
- Like the Thanator of Pandora I pursue comprehension by atacking from any possible side of the tree. --DeeperQA (talk) 04:51, 4 September 2011 (UTC)
- During the mathematical conversion of mass to energy you will get the same ratio but in the world of physics where time has infinite increments the conversion will show the amount of mass decreasing while the amount of energy is increasing for each infinitesimal increment of time. Each particle that is revealed (so far) resulting from a proton/proton collision has revealed itself as being at a stage or point of incomplete mass to energy conversion such that the ratio of mass and energy is not the same for each particle - according to the diagram. --DeeperQA (talk) 14:40, 4 September 2011 (UTC)
- What you said above makes little sense but you are probably thinking of the relationship which relates the energy E, the REST mass m, and the momentum p, as opposed to the relationship which relates the energy E to the TOTAL mass m also known as the relativistic mass. Dauto (talk) 16:34, 4 September 2011 (UTC)
- The diagram may or may not have been referring to or addressing relativistic mass. My faint recall is that it was not but rather to invariant mass only; the idea being that each particle was represented by invariant mass and proportionate energy according to Einstein's invariant mass-energy equation. --DeeperQA (talk) 21:53, 4 September 2011 (UTC)
- What you said above makes little sense but you are probably thinking of the relationship which relates the energy E, the REST mass m, and the momentum p, as opposed to the relationship which relates the energy E to the TOTAL mass m also known as the relativistic mass. Dauto (talk) 16:34, 4 September 2011 (UTC)
- DeeperQA: Exactly how is that relevant to your initial question? Plasmic Physics (talk) 12:48, 4 September 2011 (UTC)
- No one has yet seen any of the particles from a proton/proton collision occupy the same exact space at the same moment of time - that I am aware of. --DeeperQA (talk) 15:03, 4 September 2011 (UTC)
- That is easily explained by the extremely low probability of it happening, ignoring every other reason. Plasmic Physics (talk) 22:57, 4 September 2011 (UTC)
- Dauto: I'm singling out photons, because they are massless. Plasmic Physics (talk) 05:41, 4 September 2011 (UTC)
- Photons are not the only massless particles.
- Energy is a property of all particles. There is no reason to single out massless particles. Dauto (talk) 06:58, 4 September 2011 (UTC)
- Dauto: I'm singling out photons, because they are massless. Plasmic Physics (talk) 05:41, 4 September 2011 (UTC)
- Your second point: that is why I refered to massed and massless particles. I didn't intentionally single out either one, I just covered both bases. I am only aware of photons being massless, as far as I've learned; I used photons to represent massless particles. Plasmic Physics (talk) 07:27, 4 September 2011 (UTC)
- Gluons and Gravitons are also massless, and neutrinos are almost massless. At high enough energies the Electroweak symmetry is restored and the photon, W and Z bosons are replaced by isospin and hypercharge bosons which are also massless. At such high energies quarks and leptons are likely all massless as well.
HOW DO DOGS FIGHT
[edit]do they just bite each other a lot or do they use their paws and claws, like cats do? — Preceding unsigned comment added by Fran Cranley (talk • contribs) 02:31, 4 September 2011 (UTC)
- They use their paws to pin or push flesh held by their teeth. --DeeperQA (talk) 02:38, 4 September 2011 (UTC)
- There are many dogfight videos. In this one the dogs are equally matched but one rolls on its back to indicate submission, even freezing when the upper dog takes a potentially lethal neck bite. Cats fight differently by staring confrontation, batting with their paws and aiming to hold the opponent in a prolonged death bite. Cuddlyable3 (talk) 14:22, 4 September 2011 (UTC)
- They use their paws to pin or push flesh held by their teeth. --DeeperQA (talk) 02:38, 4 September 2011 (UTC)
Coordinates, part 2
[edit]Suppose that you are in the process of planning a long-distance overwater flight on a Great Circle route and don't have access to GIS software for some reason (perhaps because you are in a remote third-world country without Internet access or even a reliable electrical power supply). Is Vincenty's method the only satisfactory way to calculate the courses to fly, or is there a simpler way? Not that I have anything against Vincenty's method, but if there is one that's less tedious, I'd like to know about it. 67.169.177.176 (talk) 02:37, 4 September 2011 (UTC)
- Take a globe and stretch a piece of yarn between the two points. Then fly the course the yarn takes. Whoop whoop pull up Bitching Betty | Averted crashes 15:09, 4 September 2011 (UTC)
- Or a dressmaker's tape measure. The only downside is, this method requires accurate graphical transfer of the course to the map in order to calculate the course. After all, on a great circle route, the course does change from start to finish. 67.169.177.176 (talk) 23:02, 4 September 2011 (UTC)
- If you approximate the Earth as a sphere, the distance to your target is the radius of the Earth times the angle (in radians) between vectors from the center of the Earth to the current and target locations. In other words, it's Re cos−1 (A·B), where A and B are normalized vectors from the center of the Earth to the current and target locations respectively. The bearing to your target is the angle between two planes, one containing the center of the Earth, your current location, and the target location, and the other containing the center of the Earth, your current location, and the north pole. In other words, it's cos−1
(A×B)·(A×N), where N is anormalizedvector from the center of the Earth to the north pole. -- BenRG (talk) 05:05, 4 September 2011 (UTC)- And that will get you within radio range of your destination. Thanks, BenRG, this was very helpful. 67.169.177.176 (talk) 05:45, 4 September 2011 (UTC)
- Just to make sure: the above technique would give me the initial course; to calculate the final course (right before the destination), I'd have to replace (AxB) with (BxA) and (AxN) with (BxN), and take the reciprocal course of what I get. Is that correct? 67.169.177.176 (talk) 23:02, 4 September 2011 (UTC)
- If you have access to a Lambert conformal conic map of the relevant region, you can use it (and a protractor) to plot your course. Deor (talk) 11:31, 4 September 2011 (UTC)
- And that will get you within radio range of your destination. Thanks, BenRG, this was very helpful. 67.169.177.176 (talk) 05:45, 4 September 2011 (UTC)
Hurricanes versus wirlpools
[edit]Why do hurricanes suck upward at the center whereas whirlpools suck downward? --DeeperQA (talk) 02:45, 4 September 2011 (UTC)
- Because hurricanes (and tornadoes) are ascending vortices and whirlpools are descending vortices. Simple as that. 67.169.177.176 (talk) 03:06, 4 September 2011 (UTC)
- The one exception to what I just said, though, is the microburst, which is essentially an upside-down tornado and sucks downward. You don't tend to hear about those too much, though, because they're mainly an aviation hazard. 67.169.177.176 (talk) 03:11, 4 September 2011 (UTC)
- He has established that, he wants to know why. Plasmic Physics (talk) 03:09, 4 September 2011 (UTC)
- Because hurricanes/tornadoes are formed from columns of warm air that tend to rise. On the other hand, whirlpools are caused not by temperature differences, but by submerged obstacles mechanically disrupting the flow of water (e.g. in the lee of submerged rocks), which tends to cause downdrafts (sorry for the aeronautical terminology) as water rushes in to fill the void. Think about it: the submerged rock blocks part of the water flow, causing the water level to drop somewhat in its immediate lee -- and at the same time, the water flowing around the sides of it (and over the top) speeds up because of Bernoulli's principle. This causes suction to develop as the water speeds up, while at the same time, an inward and downward flow develops because of the lower water level behind the rock, and because the water flowing over the top of the rock deflects downward. That's why whirlpools always suck downward. 67.169.177.176 (talk) 03:25, 4 September 2011 (UTC)
- I don't believe that hurricanes do suck upwards at the center. At the eye, cool air from the stratosphere pours down: "A strong tropical cyclone will harbor an area of sinking air at the center of circulation". StuRat (talk) 06:17, 4 September 2011 (UTC)
- That doesn't stop the center from having the lowest pressure in the whole cyclonic system. And if hurricanes didn't suck upwards, then what would cause the lethal storm surge that always happens during a hurricane? 67.169.177.176 (talk) 22:53, 4 September 2011 (UTC)
- My understanding is that the storm surge is primarily caused by strong winds pushing the water onto the land, not low pressure sucking the water upwards. —Bkell (talk) 00:29, 5 September 2011 (UTC)
- It's both. Yes, the eye does suck the water upward a few feet, but it doesn't suck the air up, it sucks it down. If you think about it, a low pressure region at the surface of the water must suck both the water up and air down. StuRat (talk) 03:12, 5 September 2011 (UTC)
cardio endurance
[edit]If your lower body gets tired easily whilst running, what is the best way to challenge and improve your cardio endurance? 90.209.163.216 (talk) 09:26, 4 September 2011 (UTC)
- Sprint or long distance? If you find a work plan for endurance training your cardio performance will improve at the same time. It sounds as if your running muscles still need more excise. If they are not simply tired but ache, then more exercise will help them deal with the accumulation of lactic acid. A good plan should start of at a level you can achieve without straining and progressively build up. The 5BX plan will improve your overall fitness to a high level as well and it does it in small steps. --Aspro (talk) 10:18, 4 September 2011 (UTC)
- Agreed. However, if there is actually something wrong with your legs that prevents running, another good cardio exercise is swimming laps. You can use your legs while swimming, but also have the option of using arms only. StuRat (talk) 03:09, 5 September 2011 (UTC)
- I like to use a cross trainer because it exercises upper and lower body muscles at the same time, and I find it more pleasant than just running. Also, I don't have a TV at home, but there are five sets in front of the cardio equipment at our companies fitness room ;-). I do swim and cycle for fun, but I find it harder to keep motivated for that than for a heart-rate controlled set-time exercise. --Stephan Schulz (talk) 14:57, 5 September 2011 (UTC)
- I've found the plan in "'Run less, run faster," to be very helpful in improving my speed and endurance. The book prepares people for a marathon, but starts with a "couch potato to 5K plan in 12 weeks. Edison (talk) 16:48, 5 September 2011 (UTC)
Meningismal pain
[edit]Is there a term called meningismal pain ? It is used in the same sense as meningismus pain. aniketnik 13:09, 4 September 2011 (UTC) — Preceding unsigned comment added by Aniketnik (talk • contribs)
- The adjective meningitic is appropriate. Cuddlyable3 (talk) 13:52, 4 September 2011 (UTC)
- I think the more common adjective is meningial. Looie496 (talk) 14:28, 4 September 2011 (UTC)
- That should be spelled meningeal. Cuddlyable3 (talk) 20:43, 4 September 2011 (UTC)
- I think the more common adjective is meningial. Looie496 (talk) 14:28, 4 September 2011 (UTC)
I agree with you Cuddlyable3. The word is meningismus and I came across something which was similar to meningismus and sounded like meningismal, but you have put the appropriate way of representing the things.
10:02, 5 September 2011 (UTC) — Preceding unsigned comment added by Aniketnik (talk • contribs)
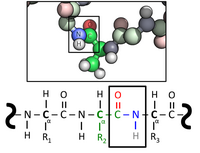
I created this image for the Protein article. I realized after the fact that I had drawn alpha carbons that alternated D and L isomers in the first revision, and have since corrected it. Would anyone care to glance at it and see if they can spot anything else? I think it's fine now, but I just want to make sure. Thanks I(q) = User(q)·Talk(q) 15:21, 4 September 2011 (UTC)
- Note: I've reduced your thumbnail, as it was taking up an unreasonable amount of space. People who want to help should simply click on the image to see the full-size version. Looie496 (talk) 16:35, 4 September 2011 (UTC)
- Looking at it, some cosmetic issues come to mind:
- The model is backward compared to the structure, which seems like no big deal to us, but might confuse the reader who doesn't know what a peptide bond is.
- The peptide bonds in this model seem a lot shorter than the other C-N bonds, by comparison to the existing illustration. Now I haven't actually looked up the bond lengths and I know they really are shorter by some amount, but you should double check.
- I understand you want to stress one peptide bond unit, but I think the other nitrogens and carbons need a bit more blue and green in their shading so people can recognize what they are.
- Hope this helps... Wnt (talk) 17:34, 4 September 2011 (UTC)
- It does help. Thank you. I'll revert back to the original illustration while I address these issues. I(q) = User(q)·Talk(q) 17:44, 4 September 2011 (UTC)
- Could you clarify in the second point whether model refers to the chemical formula or the three-dimensional representation? (I'm assuming it refers to the chemical representation since the three-dimensional portion is from an actual pdb file so it's unlikely that the bond lengths would be off there) Thanks I(q) = User(q)·Talk(q) 17:49, 4 September 2011 (UTC)
- I meant the three-dimensional representation. I think the effect is an optical illusion that results because the balls are large, but still have a little bit of rod connecting them in the nonpeptide C-N bonds. Wnt (talk) 18:16, 4 September 2011 (UTC)
- Ah. I see what you mean. The ball-and-stick representation probably doesn't help much either. Would sticks or perhaps a space-filling/sphere model be more clear? I(q) = User(q)·Talk(q) 18:20, 4 September 2011 (UTC)
- I pretty much like the figure under peptide bond, though of course your new image collating the chemical formula and the image should be an improvement. Wnt (talk) 19:21, 4 September 2011 (UTC)
- I agree, File:Peptide_bond.png already clearly illustrates the connection at this part of the molecule, which seems to be completely invisible in this proposed alternative. Space-filling (vdw) would be even worse in this respect. Also, Wnt's earlier comment is a critical one (that applies to both File:Peptide_bond.png and the proposed replacement): if you're using parallel structures to make an analogy between them or clarify their relationship, it's important that they be as similar as possible, especially in the critical area being discussed. The reversed chain-direction or changed orientation of the structure is not a good thing here. The solution is probably just to rotate the 3D image 180° in the plane of the screen, so that it becomes N-to-C and carbonyl pointing up. DMacks (talk) 14:14, 5 September 2011 (UTC)
- Thank you for your feedback. I'll upload a revised figure
tonightsoon. I(q) = User(q)·Talk(q) 14:19, 5 September 2011 (UTC)
- Thank you for your feedback. I'll upload a revised figure
- I agree, File:Peptide_bond.png already clearly illustrates the connection at this part of the molecule, which seems to be completely invisible in this proposed alternative. Space-filling (vdw) would be even worse in this respect. Also, Wnt's earlier comment is a critical one (that applies to both File:Peptide_bond.png and the proposed replacement): if you're using parallel structures to make an analogy between them or clarify their relationship, it's important that they be as similar as possible, especially in the critical area being discussed. The reversed chain-direction or changed orientation of the structure is not a good thing here. The solution is probably just to rotate the 3D image 180° in the plane of the screen, so that it becomes N-to-C and carbonyl pointing up. DMacks (talk) 14:14, 5 September 2011 (UTC)
- I pretty much like the figure under peptide bond, though of course your new image collating the chemical formula and the image should be an improvement. Wnt (talk) 19:21, 4 September 2011 (UTC)
- Ah. I see what you mean. The ball-and-stick representation probably doesn't help much either. Would sticks or perhaps a space-filling/sphere model be more clear? I(q) = User(q)·Talk(q) 18:20, 4 September 2011 (UTC)
- I meant the three-dimensional representation. I think the effect is an optical illusion that results because the balls are large, but still have a little bit of rod connecting them in the nonpeptide C-N bonds. Wnt (talk) 18:16, 4 September 2011 (UTC)
- Could you clarify in the second point whether model refers to the chemical formula or the three-dimensional representation? (I'm assuming it refers to the chemical representation since the three-dimensional portion is from an actual pdb file so it's unlikely that the bond lengths would be off there) Thanks I(q) = User(q)·Talk(q) 17:49, 4 September 2011 (UTC)
- It does help. Thank you. I'll revert back to the original illustration while I address these issues. I(q) = User(q)·Talk(q) 17:44, 4 September 2011 (UTC)
- Looking at it, some cosmetic issues come to mind:
How about this version?

List of changes:
- Both formula and structure now are represented N --> C
- Selective desaturation has been minimized
- R group specifically labeled as R = CH3
- Spheres have been reduced to 0.3*vdw to make bonds more visible.
- Alpha carbon is now explicitly labeled in 3d representation.
- Caption now refers to amide linkage instead of peptide bond to justify inclusion of the carboxyl oxygen and the amide proton in the highlighted region
Hopefully this version is an improvement over the one I uploaded the other day. I(q) = User(q)·Talk(q) 14:56, 5 September 2011 (UTC)
- I think this is definitely looking like a good figure. One quibble - I'm not sure why you made the carbons to the right of the bonded nitrogen fully green, since they're not green in the formula. (It looks like you successfully made the other "gray" carbons and nitrogens more recognizably green and blue, BTW, without any risk of confusing them with the highlighted carbons and nitrogens) Also, I don't think that the legend change to "amide linkage" is strictly necessary - according to that article, and my recollection, the peptide bond is the same thing as an amide linkage (merely used in a biochemical context) and implies these other components are present. It's true that I myself used "peptide bond" thinking only of the C-N above, but of course it can't exist without the carbonyl as part of the resonance. Wnt (talk) 18:26, 5 September 2011 (UTC)
- I've desaturated the carbons to the right of the highlighted region and agree about the caption. --I(q) = User(q)·Talk(q) 01:30, 6 September 2011 (UTC)
- It's looking good to me. Wnt (talk) 22:40, 6 September 2011 (UTC)
- Thank you for your help. I(q) = User(q)·Talk(q) 12:37, 7 September 2011 (UTC)
- It's looking good to me. Wnt (talk) 22:40, 6 September 2011 (UTC)
- I've desaturated the carbons to the right of the highlighted region and agree about the caption. --I(q) = User(q)·Talk(q) 01:30, 6 September 2011 (UTC)
- I think this is definitely looking like a good figure. One quibble - I'm not sure why you made the carbons to the right of the bonded nitrogen fully green, since they're not green in the formula. (It looks like you successfully made the other "gray" carbons and nitrogens more recognizably green and blue, BTW, without any risk of confusing them with the highlighted carbons and nitrogens) Also, I don't think that the legend change to "amide linkage" is strictly necessary - according to that article, and my recollection, the peptide bond is the same thing as an amide linkage (merely used in a biochemical context) and implies these other components are present. It's true that I myself used "peptide bond" thinking only of the C-N above, but of course it can't exist without the carbonyl as part of the resonance. Wnt (talk) 18:26, 5 September 2011 (UTC)
Eye of a hurricane
[edit]How can the eye of a hurricane have the LOWEST pressure in the hurricane, when the air in the eye is moving DOWNWARDS and the air in the rest of the hurricane is moving UPWARDS? Shouldn't the eye have the HIGHEST pressure in the hurricane, not the lowest? Whoop whoop pull up Bitching Betty | Averted crashes 15:32, 4 September 2011 (UTC)
- There are other things to consider besides pressure - namely Coriolis, centrifugal force, and friction with the surface. Dauto (talk) 15:45, 4 September 2011 (UTC)
- Less esoteric and more answer, please. Whoop whoop pull up Bitching Betty | Averted crashes 16:06, 4 September 2011 (UTC)
- this page has a picture which sketches the direction of the forces present in a hurricane. Inside the eyewall ,due to the small radius (and high wind speed), the combination of Coriolis and centripetal forces overwhelms the pressure force forcing air to move outwards causing air to sink at the eye. Dauto (talk) 16:09, 4 September 2011 (UTC)
- I meant why does it have the lowest pressure, not why does the air sink. Whoop whoop pull up Bitching Betty | Averted crashes 16:11, 4 September 2011 (UTC)
- The combination of Coriolis and centrifugal forces force the air outwards reducing the total amount of air in the column at the center. Less air in the column translates as low pressure at the surface. Dauto (talk) 16:19, 4 September 2011 (UTC)
- I don't know the answer, but reading hurricane, it sounds like the driving force is that when the humid air flowing in toward the eyewall starts to rise upward, the water in it condenses, giving it extra heat, which makes it even more buoyant. That makes it overshoot high up into the atmosphere. And because the Coriolis and centrifugal* forces conspire to keep this air rising up near the eyewall from going straight at the center, it looks like it instead cools high up and comes back down again further out from the eye, and so it's actually removing pressure from the center rather than adding it. Look at the figure in that article to see what I mean. Though I could be wrong...
- * I wouldn't say "centripetal force" here, because Coriolis and centrifugal forces are both fictitious depending on the Earth's and hurricane's frames respectively. And there is no centripetal force (or rather, not enough of one) - otherwise air wouldn't be getting sucked down into the eye from above. Wnt (talk) 17:51, 4 September 2011 (UTC)
- In short, the eye of the hurricane is never actually 'filled', thus: low pressure.-- Obsidi♠n Soul 19:00, 4 September 2011 (UTC)
- Also note that when the cool air sinks in the center, it doesn't just accumulate and build up pressure. It spreads out at the surface of the water, passing under the eye wall, rapidly warming and becoming humid, and then it rises back up. It's a huge, rotating convection cell. StuRat (talk) 03:05, 5 September 2011 (UTC)
Fissioning a hydrogen atom
[edit]Is this possible? I'm pretty sure the answer is no, but I just want to be sure. If you were to strike the nucleus of a hydrogen atom with a high energy neutron, what would occur? ScienceApe (talk) 20:47, 4 September 2011 (UTC)
- What would that mean? Fission is the splitting of atomic nuclei. A hydrogen nuclei is made of of a single proton, so there is nothing to split. You could try and split the proton into its constituent quarks, but that's not fission. You could try and fission heavier isotopes of hydrogen, but since there is only one proton to go around, you couldn't end up with two nuclei at the end so it doesn't sound like fission to me. --Tango (talk) 21:04, 4 September 2011 (UTC)
- Fissioning heavier isotopes of hydrogen would produce some extra neutrons that would eventually decay into protons (plus other stuff) and those extra protons would form extra hydrogen atoms so in a sense you would be splitting one hydrogen atom into two (or more) hydrogen atoms. Dauto (talk) 22:17, 4 September 2011 (UTC)
- You'd basically get a game of billiards. :) The neutron would bounce off the hydrogen's proton and each would move away at half the original speed of the striking neutron. This is why water is useful for absorbing neutron radiation. It may ionize the hydrogen though, leaving it pretty much, a lonely proton.-- Obsidi♠n Soul 21:24, 4 September 2011 (UTC)
- A quick search finds that deuterium was formed by n-p fusion in the early universe.[1] It would be nice if someone with a good knowledge of the topic would start that article. But I doubt you can break apart a proton with a neutron unless the two actually get fused into a quark-gluon plasma, which as much as I understand has only been done with much larger nuclei. Wnt (talk) 21:55, 4 September 2011 (UTC)
- The article already exists. It's called nucleosynthesis. Dauto (talk) 22:17, 4 September 2011 (UTC)
- Also neutron capture isn't really likely with high energy neutrons. The neutrons have to be thermalized first, i.e. bounced around several nuclei (moderators) before they can slow down enough to be grabbed by a Hydrogen proton, turning protium into deuterium, deuterium to tritium, etc.-- Obsidi♠n Soul 22:36, 4 September 2011 (UTC)
Confusing enzyme kinetics picture
[edit]I asked a question on the talk page for Enzyme kinetics about one of the pictures that appears on that page. It was probably a silly question, or maybe no one's yet chanced upon it, and I was hoping I might get some answers here. Thanks! Leonxlin (talk) 21:03, 4 September 2011 (UTC)
- I responded there. It's not a change over time, despite the green arrows. Wnt (talk) 22:02, 4 September 2011 (UTC)

2. Increasing the number of dots increases the speed at which Pacmans eat dots and poop poop-dots. As they now have an easier time of finding dots near them.
3. Increasing it further means there are now way too many dots, Pacmans can only eat a certain amount of dots at a time, and thus the rate of poop-dot production plateaus to how fast Pacmans eat dots and poop poop-dots.
- Think of substrates as lovers separated by a high electric fence. They stand on opposite sides of a row of gates and now long to reunite. Think of enzymes as the people manning the rows of gates who have to stamp something official on the foreheads of each person as they pass through the gate to meet their soulmates on the other side.
- In normal reactions which does not involve catalysts, the rate of reactions is pretty much straightforward. i.e. there is no gate and no gatekeeper. The reactants would simply rush towards each other and um.. kiss and hug and stuff.
- In catalyzed reactions which does not change the equilibrium of reactants and their products (e.g. A + B → AB + C → A + BC, where A is the catalyst), they now have to pass the gatekeeper. When there are few substrates, they rate at which they pass the gate would still be straightforward, as they can simply pick different gates. Increasing the amount of substrates can increase the speed of reaction as people would then find an empty gate faster. The rate of reaction is thus linearly increasing.
- But at higher concentrations, the gates would fill up (saturated) and the rate would then be entirely dependent on how fast the gatekeepers can stamp people on the foreheads. Thus the rate reaches maximum, flattening to a plateau.
- Note that this only applies to catalysts which are not consumed or otherwise deactivated in the reaction. In catalysts which are destroyed by each reaction (e.g. AB + C → ABC + D → A + B + CD, where AB is the catalyst), the rate will depend on how many catalysts there are from the get-go, and increasing the amount of substrates will have no effect on the speed of the reaction whatsoever.
- That's basically it. :D I couldn't think of better analogies. :P If that doesn't work, see pic of Pacmans and dots. -- Obsidi♠n Soul 22:19, 4 September 2011 (UTC)
- I've taught it using various types of construction/service/contract employees as analogy. For example, ordinarily, most of the local fire crews here sit around doing pretty much anything but fighting fires because there are so few fires most of the time (when one breaks out, one of the many available idle crews is immediately dispatched and then returns to idle after extinguishing it)--rate is limited by low amount of substrate. If fire calls start coming in a bit more rapidly, they can likely still be reached and extinguished promptly, but more of the crews are in action--there is available enzyme capacity to handle the increased amount of substrate. On Devil's Night, there is so much arson that all the crews are constantly in action, and some calls that come in can't be dispatched immediately because there are no idle crews--substrate overwhelms enzyme and enzyme becomes the rate-limit. Easily tunable for any similar service-personnel that's in the local news. DMacks (talk) 11:37, 6 September 2011 (UTC)



