Wikipedia:Reference desk/Archives/Science/2009 November 30
| Science desk | ||
|---|---|---|
| < November 29 | << Oct | November | Dec >> | December 1 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
November 30
[edit]Do microwaves disrupt Wi-Fi signals?
[edit]Do microwaves disrupt Wi-Fi signals? The other day I was connected to a weak signal on my laptop. I was sitting about fifteen feet away from a microwave oven, and the instant it was turned on, I lost my signal and couldn't regain it until the microwave finished cooking. Is it possible for the microwaves to disrupt the signals, or was this just an odd coincidence? Also, if a closed microwave oven can disrupt Wi-Fi signals from fifteen feet away, can those escaped microwaves be harmful to people? Livedtype (talk) 01:08, 30 November 2009 (UTC)
- Microwave ovens use frequencies around 2.45GHz. The 802.11 variety of WiFi uses frequencies around 2.4GHz - so, yep - any leakage from your microwave will interfere with your WiFi. So, no surprises there. Now - is your WiFi going to cook you? No - it produces at most 100 milliWatts - and even the smallest microwave oven produces 500 Watts - 5,000 times more energy. US Federal standard limits the amount of microwaves that can leak from a microwave oven to 5 milliwatts per square centimeter - but there is a lot more than 100 square centimeters on the surface of your oven - so it can easily overwhelm your puny WiFi connections. Try repositioning your oven - or your WiFi gizmo's - or try switching them to a different frequency band (you'll need to consult the manual for your WiFi router to figure out how to do that). SteveBaker (talk) 01:25, 30 November 2009 (UTC)
- Insert obligatory xkcd reference here. Zunaid●for your great great grand-daughter 08:43, 30 November 2009 (UTC)
Artificial black hole
[edit]Would it be (theoretically) possible for humans to create an artificial micro black hole by encapsulating a piece of non-fissile matter (say, a small lead sphere) with extremely powerful, shaped nuclear charges, which are then detonated in order to compress the sphere beyond its Schwarzschild radius and trigger implosion? --Kurt Shaped Box (talk) 01:36, 30 November 2009 (UTC)
- The following may help: Micro black hole#Can we produce micro black holes? Red Act (talk) 02:08, 30 November 2009 (UTC)
- Is this about the Large Hadron Collider, which started collisions on the day of posting? ~AH1(TCU) 01:43, 2 December 2009 (UTC)
- Nope. Didn't even realize that it had. --Kurt Shaped Box (talk) 21:11, 2 December 2009 (UTC)
Question
[edit]Suggest possible diagnoses given the following symptoms; if there is a symptom that does not fit, note this:
- Diarrhea
- Coughing
- Low fever/chills
- Fatigue
- Slight headache
- "Stuffed up" ears
Use your own words. Do NOT copy out of the textbook or any other source. This is PLAGIARISM and will result in a lowered/FAILING grade. 76.228.195.148 (talk) 02:29, 30 November 2009 (UTC)Tristan (at my mom's compy)
 Please do your own homework.
Please do your own homework.- Welcome to Wikipedia. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. --Tagishsimon (talk) 02:30, 30 November 2009 (UTC)
- OK, OK, I admit I was tryin to put one over on the man there :). I've so far considered influenza, but that doesnt fit low fever, 'cause influenza has high fever. Common cold doesnt fit because I thought that didn't cuase diarrhea. Stuffed up ears has me stumped, because I don't even know the name for that symptom. TOnsilitis doesn't fit the symptoms in the rear, and app'itis doesn't fit anything in the top Help? P.S.:The professor said something about no zebra, and he gave a hint that coughing was wet This is due tomorrow help! 76.228.195.148 (talk) 02:50, 30 November 2009 (UTC)Tristan
- Keep in mind that people with a condition can have varying degrees of a symptom, which may also vary over time (i.e. don't be too dogmatic). Stuffed up ears sounds like Eustachian tube dysfunction. You seem to be thinking about many reasonable possibilities - you might expand on things that are similar to your strongest suspicion, whatever you decide that is. -- Scray (talk) 03:06, 30 November 2009 (UTC)
- Alrgiht, I have a new thought: Could it be Swine flu?? Swine flu explains most symtoms, exept the ears (and I think the prof meant like acute stuffed up, not like from birth, or recurring). OK, Im almost done with this paper (Thank God; it's past 9pm where I am at, and I need to get up before 5!). All I need now is prognoses. If it's swine flu, assuming the subject is not immuno-compromised, I think prog is something along the lines of "good chance of full recovery", no? 76.228.195.148 (talk) 03:22, 30 November 2009 (UTC)tristan
- Wait, I got a email from the prof. It's copied below
- Keep in mind that people with a condition can have varying degrees of a symptom, which may also vary over time (i.e. don't be too dogmatic). Stuffed up ears sounds like Eustachian tube dysfunction. You seem to be thinking about many reasonable possibilities - you might expand on things that are similar to your strongest suspicion, whatever you decide that is. -- Scray (talk) 03:06, 30 November 2009 (UTC)
- OK, OK, I admit I was tryin to put one over on the man there :). I've so far considered influenza, but that doesnt fit low fever, 'cause influenza has high fever. Common cold doesnt fit because I thought that didn't cuase diarrhea. Stuffed up ears has me stumped, because I don't even know the name for that symptom. TOnsilitis doesn't fit the symptoms in the rear, and app'itis doesn't fit anything in the top Help? P.S.:The professor said something about no zebra, and he gave a hint that coughing was wet This is due tomorrow help! 76.228.195.148 (talk) 02:50, 30 November 2009 (UTC)Tristan
Hey, Med-I students. Regarding the assignment (I'm assuming most of you are done with it since you've had all Thanksgiving to do it), I want to give it a little...twist. For extra credit, talk about what the prognosis would be if our patient has been sick for a while (more than a week or two, but not as long as...let's say two months). I am offering 20! extra credit points for this—in other words, about a fourth of the assignment. But the real twist is, you don't get an extension! It's due tomorrow, ec or not.
I really need this credit. Help again? —Preceding unsigned comment added by 76.228.195.148 (talk) 03:30, 30 November 2009 (UTC)
- Could be lupus? 74.105.223.182 (talk) 03:48, 30 November 2009 (UTC)
- Which part of "We are not allowed to do your homework" do you not understand? No, no, NO! SteveBaker (talk) 04:08, 30 November 2009 (UTC)
- Backed up by "who wants to be treated by a doctor who got his qualifications by cheating?" AndrewWTaylor (talk) 11:17, 30 November 2009 (UTC)
- In case repetition helps - we provide nudges when people get stuck and ask for a little assistance, but you're past that now. -- Scray (talk) 05:11, 30 November 2009 (UTC)
- I diagnose it as "laziness and procrastination", waiting until the last minute and hoping someone will tell you the answer rather than actually reading your available class and reference materials to learn about the topic. Here's a freebie: the goal of this assignment is the information contained in the answer itself and also how to figure it out. DMacks (talk) 14:35, 30 November 2009 (UTC)
- Q: What do you call the guy who cheated his way through medical school? A: "ɹoʇɔop". --Sean 15:16, 30 November 2009 (UTC)
- Have you considered that a person can have more then 1 disease at the same time? Googlemeister (talk) 16:28, 30 November 2009 (UTC)
- www.webmd.com has a symptom checker that you can put in your list of symptoms and it will spit back a list of possible diseases. So your list runs the gammut of the common cold to Cryptococcosis, a fungus that grows on pidgeon droppings! Your professor's comment about a zebra was probably the old doctors adage: "When you hear hoofbeats, think horses not zebras." Which means if you see this list of symptoms, don't jump on the most complicated because you are probably wrong (we have an article on it [1]. Livewireo (talk) 16:51, 30 November 2009 (UTC)
- Have you considered that a person can have more then 1 disease at the same time? Googlemeister (talk) 16:28, 30 November 2009 (UTC)
- Sounds like an episode of House to me. Obviously you need to put the patient in an MRI scanner to suck the iron out of their tattoo ink while your coworkers break into their house and locate their stash. Meanwhile your prof can practice his yoyo or spin a plate on the end of his walking stick. And remember - (a) it's never lupus and (b) the patient's prognosis is favourable unless it's the end of series finale ! Gandalf61 (talk) 17:05, 30 November 2009 (UTC)
Di-t-boc
[edit]It seems kind of like bad atom economy to make it symmetrical -- why not use a halide leaving group in the presence of some base? John Riemann Soong (talk) 02:32, 30 November 2009 (UTC)
- If you think of Di-tert-butyl dicarbonate the problem is that the substitution of one t-Bu-O by one Cl leads to a unstable molecule. The substitution of the t-Bu-O by CH3 makes the molecule inreactive. The diester of carbonic acid just in the midle. It is not especially green, but this word is of no specially interest in the science lab and the industry would avoid a that expensive protection group.--Stone (talk) 10:56, 30 November 2009 (UTC)
- Wait it's an anhydride-type species, right? Putting a halide has a stronger activating effect than anhydride, but don't we usually use non-aqueous solvents when using t-boc? (I mean, aqueous solvents will hydrolyse t-boc too...). Or is it just cheaper to produce the monomer and dimerise it with some P2O5? John Riemann Soong (talk) 17:31, 30 November 2009 (UTC)
1,3 carbon overlap in carbon backbones
[edit]I was thinking about space-filling models when I realised what's been bothering me (in terms of counterintuition) though I didn't realise it till now -- in the space-filling model there appears to be possible overlap between atoms that aren't directly bonded to each other! Is this related to inductive effects or hyperconjugation? What about polarisability or if you have a big fat iodide atom on your chain?
Take the space-filling model for cyclohexane
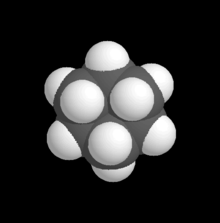
-- it appears that the six carbon atoms have some degree of overlap with each other, even if they aren't directly bonded to each other! And I've always wondered about carbon-halide bonds on rings -- with skeletal diagrams the issue seems well-concealed but sterics appears to be quite an issue! John Riemann Soong (talk) 04:13, 30 November 2009 (UTC)
- Space-filling models are just that: models. The probability density functions for the electrons do not have sharp borders, rather they have nonzero probability that extends outward indefinitely. Proximity will result in complex interactions that simple space-filling models won't capture. So, yes there is overlap in probability density among all of the atoms in a molecule. Maybe I miss your point. -- Scray (talk) 05:21, 30 November 2009 (UTC)
- (EC with below) Furthermore, space filling models are based on the covalent radius of atoms; that is the average bonding distance between atoms in a same atom bond, i.e. a C-C bond or a H-H bond. This distance isn't really related to the actual size or distribution of molecular orbitals or atomic orbitals in terms of their wave functions/probability distributions. That is, space filling models don't really represent what the molecular electron cloud will look like; if the C atoms appear to meet in the middle of the cyclohexane space-filling model, it does NOT mean that the actual electron distribution will cause "orbital overlap" (or if you prefer "molecular orbitals") between those atoms. As noted by Scray, space-filling models are models, useful for judging the overall size and shape of a molecule, but not for making any greater statements about bonding in the molecule. --Jayron32 05:30, 30 November 2009 (UTC)
- Yes, but what would you call overlap between C-R-C bonds? (95% density cutoff) ... induction? Hyperconjugation? John Riemann Soong (talk) 05:26, 30 November 2009 (UTC)
- Nothing. "A bond is at this place I draw the line or cylinder" is totally a fiction we use to explain certain things about molecules. There are not two real entities that exist and interact the way two physical objects do, because they are just probability regions. And they are regions that are part of the same molecule, so they are derived from the same functions. For example, even simple MO explanations say that there is one orbital that includes the whole A–B–C (the whole σ plane) and a second that includes "both σ bonds but not the central atom" rather than just two distinct A–B and B–C bonds. A and C certainly interact, both via sterics and electronics, depending on (as usual) their sizes and electron densities. DMacks (talk) 17:12, 30 November 2009 (UTC)
- Ok other than some elementary photochemistry and three-membered molecules I haven't really done "MO from scratch" since they pretty much abandoned that approach in my education in favour of hybridisation ... say you have a straight-chain hydrocarbon like hexane. Is there really a sigma-symmetry orbital that overlaps all six carbons (and maybe hydrogens)? So now let's put a halogen on it and let an alcohol attack by SN2... how would a nucleophile distort that orbital? Would it? John Riemann Soong (talk) 17:28, 30 November 2009 (UTC)
- Also, when you're doing something like retro-aldol cleavage ... what are you doing to those "delocalised" sigma bonds? My first intuition is that they would be some of the lowest orbitals in the molecule, but I know C-C bonds immediately formed by aldol addition (before condensation) are relatively weak. (Since there are EDG and EWG effects, are there pi interactions too?) John Riemann Soong (talk) 17:38, 30 November 2009 (UTC)
- To answer the first question, even MO theory and hybridization theory are models. As Dmacks noted, the reality is that electrons are fully mobile and non-localizable; that means that while, on average, a bond may be able to be modeled as two electrons shared between two atoms, in reality all 48 electrons in the molecule pretty much move wherever they want, randomly. Any "molecular orbitals" or "overlap between hybridized orbitals" is a model used to explain the probability distribution of the electrons in the molecule as a whole. Some models more accurately represent certain aspects of molecular shape, electron distribution, size, relative angles, etc. etc. than other models. The best models to describe the location of all electrons in a molecule would be a quantum mechanical based mathematical model, likely a Schrödinger equation. Of course, with models like this, the more accurate they become, the less intuitive and useful they are for the average user. --Jayron32 18:15, 30 November 2009 (UTC)
- Well yes, but when a radical say, extracts electrons from a bond -- those delocalised electrons must all of a sudden "localise" so they can leave? Do electrons routinely exchange between orbitals kinda like protons get exchanged in hydrogen bonding? John Riemann Soong (talk) 18:48, 30 November 2009 (UTC)
- See activated complex. When electrons "tranfer" between two molecules, they do so via a transition state called an "activated complex", which is basically a short-lived single molecule consisting of a merged complex of the two (formerly seperate) molecules. The rules for electron localizability in this activated complex are no different than for any other molecule. No single electron is transfered in a single act. The two molecules come together to form one molecule, and then seperate again; or if they stay together (such as in a Lewis acid-base reaction or in the radical bond formation you note), it doesn't matter. Also, remember that real atomic orbitals only exist in lone atoms. Once you make a molecule, you end up with molecular orbitals. There still is no need for "localization" in any of these processes. Do two drops of water "localize" their molecules when they combine to form one drop? --Jayron32 20:40, 30 November 2009 (UTC)
- Well yes, but when a radical say, extracts electrons from a bond -- those delocalised electrons must all of a sudden "localise" so they can leave? Do electrons routinely exchange between orbitals kinda like protons get exchanged in hydrogen bonding? John Riemann Soong (talk) 18:48, 30 November 2009 (UTC)
- To answer the first question, even MO theory and hybridization theory are models. As Dmacks noted, the reality is that electrons are fully mobile and non-localizable; that means that while, on average, a bond may be able to be modeled as two electrons shared between two atoms, in reality all 48 electrons in the molecule pretty much move wherever they want, randomly. Any "molecular orbitals" or "overlap between hybridized orbitals" is a model used to explain the probability distribution of the electrons in the molecule as a whole. Some models more accurately represent certain aspects of molecular shape, electron distribution, size, relative angles, etc. etc. than other models. The best models to describe the location of all electrons in a molecule would be a quantum mechanical based mathematical model, likely a Schrödinger equation. Of course, with models like this, the more accurate they become, the less intuitive and useful they are for the average user. --Jayron32 18:15, 30 November 2009 (UTC)
- Nothing. "A bond is at this place I draw the line or cylinder" is totally a fiction we use to explain certain things about molecules. There are not two real entities that exist and interact the way two physical objects do, because they are just probability regions. And they are regions that are part of the same molecule, so they are derived from the same functions. For example, even simple MO explanations say that there is one orbital that includes the whole A–B–C (the whole σ plane) and a second that includes "both σ bonds but not the central atom" rather than just two distinct A–B and B–C bonds. A and C certainly interact, both via sterics and electronics, depending on (as usual) their sizes and electron densities. DMacks (talk) 17:12, 30 November 2009 (UTC)
- Yes, but what would you call overlap between C-R-C bonds? (95% density cutoff) ... induction? Hyperconjugation? John Riemann Soong (talk) 05:26, 30 November 2009 (UTC)
- Elaborating a bit on what Jaryon said: Every set of possible nuclear coordinates has a different electronic ground state, so you have a potential energy surface for your electronic ground state in terms of nuclear coordinates. The local minima on that surface define stable compounds and the saddle-points between those valleys are the (optimal) transition-states. The motion of the nuclei is so slow compared to that of the electrons that you can regard them as instantly adjusting their energy/motion to any change of nuclear coordinates (Born-Oppenheimer approximation). You have a smooth transition from one electronic wave function to another. Transition states are tricky beasts that can't really be modeled in a simple way. The transition-state between two compounds is not typically at the halfway point in terms of coordinates, nor at the halfway point in terms of where the electrons are. E.g. I recently looked at one where the electrons were about 70% of where they'd be in the 'product' although the nuclei had only moved 20% of the way. So that's the fundamental outlook you need to have: Abandon the idea of discrete numbers of electrons in definite locations. A radical with an unpaired electron 'on' a certain atom only means it's mostly on that atom. Oxidation states are never actually integers. And so on) --Pykk (talk) 07:52, 1 December 2009 (UTC)
- Minor quibble: The "consider electrons moving nearly instantaneously on a framework of fixed nuclear positions" is more the Franck–Condon principle than Born-Oppenheimer. DMacks (talk) 08:24, 1 December 2009 (UTC)
- Elaborating a bit on what Jaryon said: Every set of possible nuclear coordinates has a different electronic ground state, so you have a potential energy surface for your electronic ground state in terms of nuclear coordinates. The local minima on that surface define stable compounds and the saddle-points between those valleys are the (optimal) transition-states. The motion of the nuclei is so slow compared to that of the electrons that you can regard them as instantly adjusting their energy/motion to any change of nuclear coordinates (Born-Oppenheimer approximation). You have a smooth transition from one electronic wave function to another. Transition states are tricky beasts that can't really be modeled in a simple way. The transition-state between two compounds is not typically at the halfway point in terms of coordinates, nor at the halfway point in terms of where the electrons are. E.g. I recently looked at one where the electrons were about 70% of where they'd be in the 'product' although the nuclei had only moved 20% of the way. So that's the fundamental outlook you need to have: Abandon the idea of discrete numbers of electrons in definite locations. A radical with an unpaired electron 'on' a certain atom only means it's mostly on that atom. Oxidation states are never actually integers. And so on) --Pykk (talk) 07:52, 1 December 2009 (UTC)
Map of the Moon
[edit]If we have satellites to map accurately every point of the surface of the Earth, so that you can even see individual lampposts on Google Earth, how come we haven't got that kind of resolution on lunar maps? —Preceding unsigned comment added by 213.229.148.222 (talk) 08:33, 30 November 2009 (UTC)
- Because we don't have a cloud of equally sophisticated satellites orbiting the moon (or any satellites for that matter). 218.25.32.210 (talk) 09:45, 30 November 2009 (UTC)
Google Earth is most likely Aerial photography at the resolutions where you can see lamp posts and cars and similar, and satalite data is only used for high level low resolution photos. Non Military satellite imagery from companies in the US are only allowed to have a resolution of 1 pixel to 0.5m (See Satellite imagery) Gunrun (talk) 10:27, 30 November 2009 (UTC)
The Mars Reconnaissance Orbiter has the camera you need and takes pictures of mars for three years now and you are far from a coverage you whant to have. The moon was uninteresting from 1972 till late 1990s and neither Clementine nor the following missions had a camera of that quality. For the maned landing they will run the camera of the Lunar Reconnaissance Orbiter for several years and they will only get coverage of the most important places.--Stone (talk) 11:02, 30 November 2009 (UTC)
- Indeed. MRO has captured images of the Phoenix lander on the surface of Mars, as well as the smaller Opportunity rover and Spirit rover, which are only about 2m across. LRO has imaged the descent stages of several of the Apollo Lunar Modules on the surface of the Moon (4m wide) and can resolve features that are 3m wide. Gandalf61 (talk) 15:13, 30 November 2009 (UTC)
Blooming of flowers.
[edit]Which chemical compound makes the flowers bloom vigourously?Is there any way to make flowers bloom in such a way? —Preceding unsigned comment added by 113.199.164.42 (talk) 11:56, 30 November 2009 (UTC)
- For some, ethylene gas might work. DRosenbach (Talk | Contribs) 13:03, 30 November 2009 (UTC)
- However, ethylene terminates the blooming of orchids (flowers wilt and drop off). On the other hand, 6-Benzylaminopurine (BAP) induces flowering in orchids [2] and possibly in other plants. See also Gibberellin --Dr Dima (talk) 03:13, 1 December 2009 (UTC)
- Florigen may be of interest, although it's not a great article. However it does cover the gist although perhaps a bit out of date i.e. we still aren't sure what substance/s induce flowering and it's one of the mysterious that interests quite a few plant biologists and there have been some recent major breakthroughs. The article also does mention that this is something that's been going back and forth, e.g. first people got all excited about the idea of a flowering hormone then after fruitless searches and some research people began to think that perhaps there wasn't a flowering hormone (i.e. florigen) after all but just a specific combination of hormones that induced flowering but that had problems too and recent evidence suggests we may have finally found the florigen. Also the name is fairly unique so you can find lots of likely useful articles by searching e.g. [3] [4] [5] Nil Einne (talk) 11:45, 1 December 2009 (UTC)
- However, ethylene terminates the blooming of orchids (flowers wilt and drop off). On the other hand, 6-Benzylaminopurine (BAP) induces flowering in orchids [2] and possibly in other plants. See also Gibberellin --Dr Dima (talk) 03:13, 1 December 2009 (UTC)
Antidepressants losing effectiveness over a decade.
[edit]If a person took the antidepressant bupropion for over a decade or two, would this lose its effectiveness of the subject becomes too used to it? --Reticuli88 (talk) 15:01, 30 November 2009 (UTC)
- In the abstract (i.e. in general) this phenomenon apparently does occur PMID 9671339, though some dispute its frequency PMID 17854252. If you're asking about an individual case (you're question is worded generally, so I'm assuming that you are not), that person should ask their doctor. -- Scray (talk) 15:31, 30 November 2009 (UTC)
- Most antidepressants are actually placebos, and only work if you believe in them [6]. So over time if you occasionally felt depressed anyway, you might start to loose faith in the drug, and then it won't work anymore. I hope that knowing this does not cause you medical problems :( I hope you are posting this on behalf of someone else, and do not tell them. Also, I should clarify: the drug does not have zero effect, it has a small one, but the majority of the effect is the placebo. Read the actual studies on it (and others) if you want the details. Ariel. (talk) 19:05, 30 November 2009 (UTC)
- Your statement is wrong. No approved antidepressant is a placebo, as they are all biochemically active compounds. The article is saying that some antidepressants appear to be no more effective than the placebo effect in the treatment of depression, which is not the same thing as saying that the drug is a placebo. Just because a compound is (or becomes) ineffective at treating its intended ailment does not mean it is inert. It could still have other effects (both positive and negative). Dragons flight (talk) 19:19, 30 November 2009 (UTC)
- Not that what is used as placebo in medical research is always inert[7]. Unomi (talk) 19:49, 30 November 2009 (UTC)
- Your statement is wrong. No approved antidepressant is a placebo, as they are all biochemically active compounds. The article is saying that some antidepressants appear to be no more effective than the placebo effect in the treatment of depression, which is not the same thing as saying that the drug is a placebo. Just because a compound is (or becomes) ineffective at treating its intended ailment does not mean it is inert. It could still have other effects (both positive and negative). Dragons flight (talk) 19:19, 30 November 2009 (UTC)
Looking for planets visibility
[edit]I would like to know what planets are visible to the unaided eye, and when they would be visible at 42 deg N for the night of April 17-18, 2010 At timezone -6 GMT. Googlemeister (talk) 19:52, 30 November 2009 (UTC)
- You can use the sky chart at Heavens Above to generate a view for any arbitrary time. Note that time zone doesn't matter for stellar/planetary observations, and the observer's latitude isn't a major concern for viewing the planets. It appears that Mercury and Venus will both be in the western sky near sunset, with a crescent moon slightly behind. Saturn and Mars should be overhead for much of the night. Jupiter may be visible pre-dawn. — Lomn 20:10, 30 November 2009 (UTC)
- And those are the 5 naked eye planets - Mercury, Venus, Mars, Jupiter and Saturn. Uranus is sometimes visible under excellent conditions if you know just where to look, but generally speaking you need binoculars for it. --Tango (talk) 21:53, 30 November 2009 (UTC)
- I have never had much luck with Mercury, but then it is only visible for a very short time right? Googlemeister (talk) 22:00, 30 November 2009 (UTC)
- Yep - it's orbitting so close to the sun that it rises and sets quite close to sunrise/sunset. That gives you only a small window of time - and since it's so close to the horizon, you're looking through a lot of atmosphere - which may still be scattering sunlight from the sun that's only just below the horizon. SteveBaker (talk) 03:39, 1 December 2009 (UTC)
- Is Mars the easiest to see with the naked eye? I seem to think I'm seeing Mars pretty often. Bus stop (talk) 03:44, 1 December 2009 (UTC)
- At peak brightness, Venus and Jupiter are the two brightest planets. "Easiest" may be subjective, though. — Lomn 03:50, 1 December 2009 (UTC)
- OK. Thanks. Bus stop (talk) 04:12, 1 December 2009 (UTC)
- Venus and Jupiter are very bright, but Mars is quite bright too at its brightest, and certainly stands out in the sky. Also, it really is red and therefore easy to identify.--Rallette (talk) 07:54, 1 December 2009 (UTC)
- OK. Thanks. Bus stop (talk) 04:12, 1 December 2009 (UTC)
- I would say that Venus and Jupiter are easier to see than Mars, the problem is that they look rather like stars to the untrained eye. Mars is clearly Mars because it is red. Venus and Jupiter can only really be distinguished from stars by the fact that they are brighter than any star (they also twinkle less due to having a larger angular diameter and are always found on the ecliptic). --Tango (talk) 12:28, 1 December 2009 (UTC)
- I would imagine that at certain times, Mars is brighter then both Jupiter and Venus depending on the positions of the planets, but it is rather rare for that to occur. Googlemeister (talk) 14:55, 1 December 2009 (UTC)
- Well, yes - because Venus (and Mercury) are closer to the Sun than the Earth, they show 'phases' rather like the moon. When Venus is directly between the earth and the sun, we're looking at the dark side and it will be essentially invisible - when Venus is on the far side of the sun, it's a lot further away - but now we're looking at the bright, sunny side - so it's pretty bright. The trouble is that Venus is at it's brightest when it's furthest away and when it's so close to the sun that we can't see it in daylight and sets too soon to be visible at night. The best time to see it is when it's about half-lit and off to the side of the sun. Mars is a different matter though. Being further out than the Earth, it's brightest when it's on the opposite side of the Earth from the Sun - and that's also when it's closest and when you have the darkest sky to see it against. So there are certainly times when Mars is brighter than Venus. SteveBaker (talk) 16:06, 1 December 2009 (UTC)
- I would imagine that at certain times, Mars is brighter then both Jupiter and Venus depending on the positions of the planets, but it is rather rare for that to occur. Googlemeister (talk) 14:55, 1 December 2009 (UTC)
- At peak brightness, Venus and Jupiter are the two brightest planets. "Easiest" may be subjective, though. — Lomn 03:50, 1 December 2009 (UTC)
- Is Mars the easiest to see with the naked eye? I seem to think I'm seeing Mars pretty often. Bus stop (talk) 03:44, 1 December 2009 (UTC)
- Yep - it's orbitting so close to the sun that it rises and sets quite close to sunrise/sunset. That gives you only a small window of time - and since it's so close to the horizon, you're looking through a lot of atmosphere - which may still be scattering sunlight from the sun that's only just below the horizon. SteveBaker (talk) 03:39, 1 December 2009 (UTC)
- I have never had much luck with Mercury, but then it is only visible for a very short time right? Googlemeister (talk) 22:00, 30 November 2009 (UTC)
- Also try YourSky, and enter your latitude, longitude, and the date and time in UTC. ~AH1(TCU) 01:26, 2 December 2009 (UTC)
- Currently, Jupiter is visible on evenings, Mars is visible later at night and appears reddish, and Venus and Saturn are visible late night to morning. On December 3, the disc of Mars will exceed ten arcseconds. The Moon will be directly above Mercury on December 18, which looks like a relatively faint object, but should rise for long enough to see; I've been successful at viewing Mercury through a telescope with its cresent. On December 20, between 7:33 and 9:08 pm, Jupiter will have a double shadow transit of two of its moons, while on that night Jupiter will be closest to Neptune in the field of view of a telescope. Uranus is located close to Jupiter, in Pices. ~AH1(TCU) 01:33, 2 December 2009 (UTC)
- And those are the 5 naked eye planets - Mercury, Venus, Mars, Jupiter and Saturn. Uranus is sometimes visible under excellent conditions if you know just where to look, but generally speaking you need binoculars for it. --Tango (talk) 21:53, 30 November 2009 (UTC)
Ray-tracing for sound not light
[edit]Would the principles of and algorithmns for ray-tracing work with sound as well as with light? Rather than creating an image, instead I would like to make a contour map of the sound intensity. 89.242.99.245 (talk) 22:35, 30 November 2009 (UTC)
- Sure. That's how Medical ultrasonography works. 169.139.217.77 (talk) 23:19, 30 November 2009 (UTC)
- Sort of. But medical ultrasonography works with wavelengths that are roughly 1000 times smaller than typical audible wavelengths, so it can deal with objects that are about 1000 times smaller before interference and diffraction become a consideration. Red Act (talk) 00:10, 1 December 2009 (UTC)
- Yes and no. They do use ray tracing for acoustics sometimes; see Ray tracing (physics)#Ocean acoustics. However, what might be a problem is that depending on the sounds and the environment involved, the wavelengths involved might not be much smaller than the sizes of the objects involved, in which case interference and diffraction would no longer be negligible effects. That's problematic because normal ray tracing algorithms don't model interference and diffraction. But that might not be an important issue if you're only dealing with high enough sound frequencies and/or large enough objects. Red Act (talk) 00:10, 1 December 2009 (UTC)
- We use acoustic ray tracing all the time in seismic imaging. Here is an overview to the method as adapted for reflection seismology. Nimur (talk) 01:12, 1 December 2009 (UTC)
- There are some tricky problems - for example, our eyes only see three frequencies of light (Red, Green, Blue) - so you can do a very good approximation for almost any frequency-dependent effect by simply tracing monochromatic red, green and blue rays separately. But for audio, our ears are very sensitive to frequency and we can distinguish very complex mixtures of them...that trick isn't going to work for sound. Also, it takes an insanely small gap to cause diffraction in light - to the extent that we can basically just ignore it. But audio diffracts as it passes through a doorway! On the plus side - the resolution of audio is so much lower than light that we don't need to trace even a tiny fraction of the number of 'rays' - if you want the same kind of calculation times - you can lavish much more CPU time on your audio rays. SteveBaker (talk) 03:36, 1 December 2009 (UTC)
- Wait, wait, wait. "our eyes only see three frequencies of light (Red, Green, Blue)"? That isn't true. Our eyes perceive different frequencies as combinations of Red, Green and Blue, but it isn't true that we only see those frequencies. We can see yellow light: we see it by perceiving it as a combination of red and green, but we still see it. I know you know loads about this, so I assume you're making some other point Steve. Could you explain a bit more? 86.166.148.95 (talk) 19:39, 1 December 2009 (UTC)
- Yes, I think Steve has made a mistake there. As astounding as it may sound, it does happen! --Tango (talk) 20:21, 1 December 2009 (UTC)
- Well, yes and no. In the context of Ray Tracing - we are going to display the resulting image on a display that very literally only displays red, green and blue light. Our eyes can't tell the difference between that and a full spectrum of frequencies. But yes - I spoke a little loosely. We have only three colors of 'sensor' in our eyes - we can't tell the difference between pure yellow light (such as you get from a sodium vapor lamp) and an appropriate mixture of pure red and pure green light - they look completely identical to our poor eyes. On the other hand - if we take the audio analogy if you play a 'D' as a pure sine-wave - it sounds very different from a chord containing a 'C' plus an 'E'. The only case where we can tell the difference between a pure color and an "optical chord" is with magenta (red+blue) - which looks a lot different from green. SteveBaker (talk) 00:45, 2 December 2009 (UTC)
- Yes, I think Steve has made a mistake there. As astounding as it may sound, it does happen! --Tango (talk) 20:21, 1 December 2009 (UTC)
- Wait, wait, wait. "our eyes only see three frequencies of light (Red, Green, Blue)"? That isn't true. Our eyes perceive different frequencies as combinations of Red, Green and Blue, but it isn't true that we only see those frequencies. We can see yellow light: we see it by perceiving it as a combination of red and green, but we still see it. I know you know loads about this, so I assume you're making some other point Steve. Could you explain a bit more? 86.166.148.95 (talk) 19:39, 1 December 2009 (UTC)
premolut n
[edit]Plz provide detail about drug premolut n —Preceding unsigned comment added by 117.254.28.61 (talk) 23:16, 30 November 2009 (UTC)
- This is a drug involved with the timing of menstruation. On wikipedia, we don't provide medical advice. You can get more information by contacting a physician or other medical provider or simply searching it on the internet. Tyrol5 [Talk] 23:21, 30 November 2009 (UTC)
- Premolut N is a brand name for norethisterone, on which we do at least have a stub article. I just now created a redirect. Red Act (talk) 00:21, 1 December 2009 (UTC)
