Wikipedia:Reference desk/Archives/Science/2007 April 15
| Science desk | ||
|---|---|---|
| < April 14 | << Mar | April | May >> | April 16 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
April 15
[edit]Stomach safety - Uncooked rice?
[edit]If you eat uncooked rice will you explode? 71.100.160.118 00:31, 15 April 2007 (UTC)
- Don't know about that, but googling "uncooked rice" "safe to eat" turns up several sites that say uncooked rice may contain harmful bacteria. Anchoress 00:36, 15 April 2007 (UTC)
- Sounds like a change in "the old wives tale" that feeing rice to birds will make them explode. The closest we've found is exploding bats I do think. [Mαc Δαvιs] (How's my driving?) ❖ 08:03, 15 April 2007 (UTC)
- There was a mythbusters episode about how to detonate a pigs stomach using soda. The stomach (without the pig) was sealed at both ends and filled with soda and water like a balloon. The expanding carbon dioxide caused the somach to rupture. —The preceding unsigned comment was added by 84.187.46.16 (talk) 12:54, 15 April 2007 (UTC).
- Eating large quantities of uncooked rice and then drinking large amount of water can be very dangerous to your health as it can cause your stomach to rupture. I think it has been used as a method of torture before. --80.229.152.246 16:41, 15 April 2007 (UTC)
- Er how? Ever soaked rice overnight for some dishes? They don't bloat as if they're cooked. --Wirbelwindヴィルヴェルヴィント (talk) 19:33, 15 April 2007 (UTC)
- Maybe that "method of torture" is like force feeding, or gavaging.
overnight in the dishes is not as hot as your stomache. [Mαc Δαvιs] ❖ 03:18, 16 April 2007 (UTC)
1 − 2 + 3 − 4 + · · ·
[edit]I didn't see it anywhere in the article, so I'm asking here... Does the subject of today's featured article, 1 − 2 + 3 − 4 + · · ·, have any useful purpose or is it just some mathmatician's noodling? Dismas|(talk) 02:14, 15 April 2007 (UTC)
- Like lots of math, it may not have an obviuos application, but is interesting nonetheless. THe fact that the series sums to 1/4 when it is divergent is especially interesting —The preceding unsigned comment was added by 88.110.28.251 (talk) 02:19, 15 April 2007 (UTC).
- It's a "counterexample" to conventional notions about convergence, suggesting that there is more depth to the subject than might be initially assumed. Nimur 04:54, 15 April 2007 (UTC)
- Yes, it is noodling, although to describe Leonard Euler as "just some mathematician" is like calling Shakespeare "just some playwright" or Mozart "just some composer". Most pure mathematics is "noodling", or at least starts out as "noodling", and is none the worse for being so. Gandalf61 08:48, 15 April 2007 (UTC)
- The importance of such puzzles may not be understood at the time the mathematician works on it - but it is amazing the number of seemingly irrelevent 'puzzles' that later became critical to human progress. A ton of work was done on prime numbers (for example) that seemed to be pure number theory with zero practical applications at the time, now have critical importance in cryptography - and hence the ability to do business safely on the Internet. I'm not aware of any important applications of the summation of this particular series - but I'd bet good money that the techniques that had to be developed in order to solve it are key to all sorts of modern tools and applications. SteveBaker 15:48, 15 April 2007 (UTC)
- As I said on the article's talk page, it's utterly pointless. Satisfying idle curiosity, as most of math does, does not benefit humanity, and so is worthless. Vranak
- "There is no branch of mathematics, however abstract, which may not someday be applied to the phenomena of the real world." (attributed to Nikolai Lobachevsky, but unsourced in wikiquote) ---Sluzzelin talk 17:52, 15 April 2007 (UTC)
- As I said on the article's talk page, it's utterly pointless. Satisfying idle curiosity, as most of math does, does not benefit humanity, and so is worthless. Vranak
- Key phrase: may someday. Vranak
- Vranak, same thing can be said about refinement in most arts, creating the music you listen too, the design of the clothes you wear, the chair you are sitting in, etc. (Geez, why am I replying to this obvious troll).
- The uncertainty of evaluating expressions like that is one of the things that brought about late 19th and early 20th century formalism. This is how we know it does not have a sum. Root4(one) 18:04, 15 April 2007 (UTC)
- Music does not satisfy idle curiosity. Chairs do not satify idle curiosity. Clothing does not satisfy idle curiosity. Vranak
- I don't know how much time you've spent doing science, Vranak, but one of the astounding things that happens is that you find yourself needing the answer to some fantastically obscure side question that's just come up in your work, and you do a literature search, to discover that, in fact, someone has not only answered that question but also published a peer-reviewed paper about it. They did this even though they had no idea whether anyone would find the result practically useful. They may never hear about the day you did actually find the result practically useful. But in the end, it was, it was.
- Doing pure research, without regard to applicability, is in some ways a luxury. And it's an expensive luxury, to boot. But it has absolutely incalculable value in terms of its ability to eventually, and in a million subtle ways, enable actual life-changing new developments, without which life as we know it could not exist.
- You can dis and refuse to pay for pure, "pointless" research if you want to, but unless you disavow yourself of all human technology, and revert to pretty much a hunter-gatherer lifestyle, you're being somewhat of a hypocrite. —Steve Summit (talk) 18:37, 15 April 2007 (UTC)
- I don't know where to begin with this. I understand what you're saying though. And I've studied some pretty extreme math in Computer Science and Astrophysics. Vranak
- I can give a concrete example of that - William Hamilton spent a large fraction of his life thinking about complex numbers (which you may recall are 'two-part numbers' having both a 'real' and an 'imaginary' part). Hamilton wondered what it would mean to have numbers with three parts instead of two. He found that three-part numbers were not very interesting - but discovered some very interesting (but entirely abstract) properties that four-part numbers have...weird - but true. These four-part numbers are called quaternions (or more rarely 'Hamiltonians' for obvious reasons). For the longest time quaternions fit Vranak's "utterly pointless" criteria. And, indeed, Hamilton was merely "Satisfying idle curiosity". OK - so fast forward about 120 years during which quaternions were more or less ignored for all practical matters. Then technologists started to represent rotations and combinations of rotations in three dimensions in the very practical fields of robotics and computer graphics. Conventionally, you'd use three angles to represent a rotation (roll, pitch and yaw) - but it turns out that this is amazingly messy - for precisely the reason that Hamilton had problems with his 'three part numbers'. Someone (I'm not sure who it was) was familiar with Hamilton's quaternions - and lo and behold, it turns out that adding a fourth number to form a quaternion results in a really elegant way to write down rotations in three dimensions - and to combine and manipulate them. That obscure and "utterly pointless" mathematical insight that Hamilton had back in the 1850's or so is now an important core principle for many computer games and for those robots you see in car plants and such. It's not at all unusual for it to take a hundred years for some piece of seemingly abstract math to become useful - but mathematicians quite often don't know in advance what will be useful and what won't - and they don't know when it'll be useful either - so they work on problems as a way of satisfying their curiosity - and years, decades or centuries later, some practical scientist or technologist will rediscover their work and make something wonderful using it. That's how mathematics is done - and we should never ever put mathematicians down for studying seemingly useless areas of their discipline. I'm no mathematician - but I surely do appreciate the work they do. SteveBaker 21:00, 15 April 2007 (UTC)
- Yeah, cars and computer games, everyone loves them. Vranak
- Actually, Vranak is correct. Most pure mathematics has no practical use (the canonical exceptions have been mentioned above, but they are few, and far between) and most pure mathematicians are motivated most of the time by idle curiosity. Hardy's A Mathematician's Apology famously justifies this stance. In a Brave New World-style dystopia, pure mathematics would be outlawed alongside art, literature and religion, as it would have no place in a purely functional society. I happen to think that the ideas behind 1 − 2 + 3 − 4 + · · · are as beautiful and elegant as Fauré's Requiem or a Canaletto, and I wouldn't want to live in a society that did not permit people to indulge in such pleasures - but that is a personal opinion, and I respect the views of those who think otherwise. Gandalf61 23:51, 15 April 2007 (UTC)
- Just because outlawing mathematics would be odious, that doesn't mean it has much value. And yes, just because I pointed this out, it doesn't make math worthless either. Vranak
I do find it ironic that Vranak is talking about a metric, that is value, and that most of mathematics does not measure up to his standards. Root4(one) 03:41, 16 April 2007 (UTC)
- Oh my god.
- Which do you think came first, conceptions of value, of worth, or people adding up numbers on an abacus? Vranak
Urea Experiment
[edit]For my senior project in high school, I recreated Wohler's Synthesis of Urea. In the reaction, lead(II) cyanate was combined with ammonia to yield ammonium cyanate which rearragned to form urea. The reaction took place in an ethanol-water mixture that was heated to 70 degrees C for 90 minutes.
I would like to go to detail in my lab report about why ammonium cyanate, an ionic compound,spontaneously rearranges to form urea, a covalent compound, and would like to prove it mathematically. I realize that the sign of ΔG must have been negative for the reaction to spontaneously occur under the given conditions. Since the reaction proceeded at high temperatures, I realize that the sign of ΔH and ΔS probably both were positive for the reaction to be spontaneous in the forward direction. My experience with gibbs free energy is limited, and all of problems I have done in class have involved gases. I was able to locate thermodynamic data for urea in the gas phase, but in my reaction the all reactants were aqueous. In addition, I couldn't find any data at all for ammonium cyanate.
I would really appreciate it if anyone could point me to a good source of data, explain to me why my book only uses gases for thermodynamics, or give other suggestions for explaining why ammonium cyanate changed to urea in my lab report. Thank you! 71.253.34.212 03:30, 15 April 2007 (UTC)purecontrol
LORAN C
[edit]how pulse and cycle matching is done in LORAN-C navigation system?thank you for any help —The preceding unsigned comment was added by Ambuj0542 (talk • contribs) 05:39, 15 April 2007 (UTC).
- Our article on LORAN might help. Splintercellguy 05:48, 15 April 2007 (UTC)
Anaerobic Respiration
[edit]CAN HUMAN BEINGS BREATHE ANAEROBICALLY FOR LONG PERIODS OF TIME? WHAT IS THE MAXIMUM TIME A NORMAL PERSON CAN BREATHE ANAROBICALLY? IF PEOPLE CAN BREATHE ANAEROBICALLY, WHY DO SOME PEOPLE STILL DROWN? —The preceding unsigned comment was added by Invisiblebug590 (talk • contribs) 06:42, 15 April 2007 (UTC).
- No, zero, and N/A. While humans are capable of anaerobic respiration, this is only to suppliment aerobic respiration in times of strenuous physical activity. It is extraordinarily less efficient than aerobic respiration, and cannot sustain a human in the absence of oxygen for any appreciable amount of time. Someguy1221 07:36, 15 April 2007 (UTC)
- Further reading here expounding on this. Anaerobic respiration can provide energy to muscles for 30 seconds to 2 minutes, but this is only in muscle cells, which might help you swim a little if you're drowning but your brain will still suffocate. Someguy1221 10:03, 15 April 2007 (UTC)
- Maybe the problem is that the inquirer is confusing anaerobic respiration (a chemical process in cells) with the act of breathing oxygen in with the lungs. alteripse 12:53, 15 April 2007 (UTC)
Dry ice safety
[edit]In our article on dry ice (actually part of the carbon dioxide article) and in other places around the web, I've seen references that dry ice is dangerous to touch because it is so cold. While dry ice is obviously pretty chilly (-78C), I think the burning danger results from it being so *dry* that it dehydrates your skin on contact. Is this correct? I can't find a source on the net, but I know from experience that if you handle CO2 with even thin gloves, it might be uncomfortably cold, but not painful if done quickly, while touching it to bare skin hurts pretty much on contact. To me, that means that it is not just temperature at work. If I am right, I'd like to find a reliable cite, so I can add it to the article. On the other hand, I'm not 100% certain that I am right, or I'd add something myself. Any help? Matt Deres 14:58, 15 April 2007 (UTC)
- It's only "dry" because solid carbon dioxide does not melt into liquid carbon dioxide. It sublimes directly into gaseous carbon dioxide. It's not dry in the sense that it contains no water.
Try touching liquid nitrogen if you want something really cold, or better yet, a piece of metal that has been cooled by liquid nitrogen. :P--Russoc4 15:12, 15 April 2007 (UTC)- (Well, it is dry in the sense that it contains no water, but you're right, that's not why it hurts, or stated another way, it doesn't hurt by dehydration, except perhaps indirectly due to freeze-drying. —Steve Summit (talk) 15:41, 15 April 2007 (UTC))
DO NOT TOUCH ANYTHING WITH YOUR BODY THAT IS BELOW THE FREEZING POINT OF WATER! Nebraska Bob 15:25, 15 April 2007 (UTC)
- Are you kidding? Do you never go outside of your (Nebraska?) house all winter for fear that you might have to touch the house's doorknob to get back in? Obviously, it's a good idea not to touch something that's below freezing and thermally massive if your skin is wet, especially if your name is Flick, but your blanket bold-faced prohibition is just plain silly. Well, unless conditions in Nebraska are a lot different than New Hampshire. ;-)
- Nope; it's just the cold that does it. Even thin gloves provide some insulation, which is vastly superior to no insulation. If you try touching something like bare metal that has been chilled with dry ice (or which comes straight from an ultralow freezer), you'll note that you get the same pain that you get from direct contact with dry ice. (I just use a double pair of latex or nitrile gloves if I need to do more than a few seconds of work in a -80 freezer; they give sufficient insulation while still leaving me some manual dexterity, but you do have to be wary of frostnip if you're going to do more than a few minutes of work.) TenOfAllTrades(talk) 15:34, 15 April 2007 (UTC)
- I have struck out User:Russoc4's
stupidly dangeroussuggestion. It is idiotic to say things like that on a public web page when our readers may not realise you are making some kind of joke (which isn't funny in any way). Liquid Nitrogen is at -195C - much, much colder than solid CO2 and it would be exceedingly dangerous to touch it - or a chunk of metal cooled by it. SteveBaker 15:36, 15 April 2007 (UTC)- In fairness, I think "stupidly dangerous" and "idiotic" are a little strong. It was a borderline comment, to be sure, but really: how many readers won't realize that in that context, the word "try" basically means "imagine"? (Of those readers, how many would be moved to actually try it? And of those, how many would have access to liquid nitrogen?) —Steve Summit (talk) 15:46, 15 April 2007 (UTC)
- I don't know - and for sure I don't recommend "the experimental approach" to finding that out. When it comes to safety matters we should have a zero tolerance policy for dumb advice...made jokingly or not. SteveBaker 20:39, 15 April 2007 (UTC)
- Well at least s/he didn't recommend liquid helium. I agree with you though. There is easily the risk a child (who shouldn't have access to liquid nitrogen but I digress) or someone with only a rudimentary understanding of English may misunderstand that Nil Einne 23:16, 15 April 2007 (UTC)
- I don't know - and for sure I don't recommend "the experimental approach" to finding that out. When it comes to safety matters we should have a zero tolerance policy for dumb advice...made jokingly or not. SteveBaker 20:39, 15 April 2007 (UTC)
- Oddly enough, touching liquid nitrogen – at least for modest lengths of time – isn't all that bad. I've done it a number of times, and it's the sort of demonstration that old physics profs (from the era before everything had a warning label) relish. When you immerse your hand in LN2, the liquid in contact with your hand boils immediately, surrounding your hand with a layer if nitrogen gas. The gas is a pretty good insulator that mostly protects your hand from contact with the rest of the very cold liquid. (This is a similar phenomenon to a water droplet 'dancing' on a very hot griddle. The layer of steam under the droplet insulates the rest of the droplet, keeping it from boiling off for an extended period of time.)
- On the other hand, if you touch something that has been chilled with liquid nitrogen but that won't boil in contact with your skin, heat will be transferred out of your hand very quickly and will potentially cause rapid injury. TenOfAllTrades(talk) 16:02, 15 April 2007 (UTC)
- In fairness, I think "stupidly dangerous" and "idiotic" are a little strong. It was a borderline comment, to be sure, but really: how many readers won't realize that in that context, the word "try" basically means "imagine"? (Of those readers, how many would be moved to actually try it? And of those, how many would have access to liquid nitrogen?) —Steve Summit (talk) 15:46, 15 April 2007 (UTC)
- I have struck out User:Russoc4's
- Things that are extremely cold (or hot) are dangerous due to the combination of their temperature and heat capacity. If something is at an extreme temperature, but has either tiny mass or a low specific heat, touching it won't hurt very much. (That's why you can get showered with sparks, from say a grinder or a sparkler, without injury, even though those sparks are basically flaming metal.)
- But dry ice obviously does have a decently high heat capacity (otherwise we wouldn't be using it for cooling things).
- If you had a piece of aerogel, or a space shuttle thermal tile, at -78°F, you could probably hold it in your bare hand without injury. —Steve Summit (talk) 15:37, 15 April 2007 (UTC) [P.S.: Probably! I'm speculating here; I really don't know for sure. "Don't try this at home, kids!"]
- it was just a joke. Actually, I meant to say "try touching liquid nitrogen if you want... to feel something cold, but I lost my train of thought and left it out. Also, one of my chemistry professors put it in his mouth! It's not like it instantly kills you. Yes, it's damn cold, but it's no more dangerous than accidentally touching a hot pot on a stove. I'm sorry if I offended you, or the reference desk. —The preceding unsigned comment was added by Russoc4 (talk • contribs) 16:17, 15 April 2007 (UTC).
- What? Stealing a tile from the space shuttle, putting it in a refrigerator at -78 C, and holding it in your hands? That's something every kid has the resources to do! --Bowlhover 16:20, 15 April 2007 (UTC)
- It's not that hard. Just wait for one to fall off (they do it all the time) and use the liquid nitrogen that was mentioned 23:20, 15 April 2007 (UTC)
- Hmmm. I think this thread needs some reliable sources. Goggling "liquid nitrogen" and "safety" turns up these safety notes on Liquid Nitrogen Demonstrations from the University of Wichita, which say:
- Teachers must stress to their students the importance of not touching frozen objects or nitrogen.
- Wear goggles whenever pouring or dumping nitrogen. Nitrogen can spatter into the eyes, and potentially blinding pieces of frozen things can fly around when we drop it.
- Use a glove and / or tongs to handle any object going into or out of nitrogen and to carry the nitrogen dewar. Gandalf61 16:47, 15 April 2007 (UTC)
I believe there is a knack to bare-handed handling of dry ice without getting hurt. It involves tossing the ice from hand to hand so that it doesn't have time to freeze any particular spot of skin. This information comes with absolutely no warranty -- I wouldn't be able to tell you how often you have to toss the ice, or whether you'll get hurt when you pick it up the first time, or anything like that, so don't come crying to me if you try it and get burned. --Trovatore 19:10, 15 April 2007 (UTC)
Thanks for the answers, everyone. We've kind of drifted a bit, but that a-okay, too. My hypothesis that dry ice essentially did a freeze drying job on your skin seems to have been an error. Checking out that article, I see that dry ice is used for that purpose, but apparently only as a cooling agent. Matt Deres 19:59, 15 April 2007 (UTC)
Hmm, well, as long as we answered your question. The point I tried to make with the liquid nitrogen is that dry ice is not that cold. You can touch it, just like you can touch regular ice made of water. If you handle it for too long, it starts to "burn", also just like regular ice. As Trovatore said, yes, you can handle it if you don't hold it for too long. The same goes for liquid nitrogen. This is how my professor puts it in his mouth, but keeping it moving around. Trust me though, neither dry ice nor liquid nitrogen are anymore dangerous than a pot of boiling water. You're natural reflexes will keep you from getting burned, and if you do, it doesn't cause serious damage. In fact, I think a burn from a pot of water hurts more, and last longer than a liquid nitrogen burn, which for me lasted minutes. --Russoc4 20:03, 15 April 2007 (UTC)
Please do not put casual references on Ref Desk to putting liquid nitrogen in the mouth, because the results could be fatal. And touching hot pans on the stove can obviously result in painful burns. Edison 20:04, 15 April 2007 (UTC)
- “We should be careful to get out of an experience only the wisdom that is in it—and stop there; lest we be like the cat that sits down on a hot stove-lid. She will never sit down on a hot stove-lid again—and that is well; but also she will never sit down on a cold one any more.”[1] —Mark Twain —The preceding unsigned comment was added by Ummit (talk • contribs) 20:42, 15 April 2007 (UTC).
Just to interject here, liquid nitrogen is not instantly fatal, and routinely physics professors slosh some around in their mouth during those great cyrogen demonstrations. [Mαc Δαvιs] ❖ 20:34, 15 April 2007 (UTC)
- Thank you Mac Davis. --Russoc4 21:14, 15 April 2007 (UTC)
- The common exaggerations of the dangers of liquid nitrogen withstanding, I'd like to share an anecodote as a counterpoint to some of the comments here. When I started my PhD at a research institute a number of years ago, my new lab-bench was next to an experienced technician's. On my 5th day in my new lab - having just got to know my new colleague - he went downstairs by himself to decant some liquid nitrogen for the lab. Exactly what happened next was never clarified, but he was found dead in a rapidly evapourating pool of liquid nitrogen just a few minutes later (and a few other lab members were injured trying to rescue him). This was a man who had been working with liquid nitrogen on a weekly basis for years. Just thought I'd add that to the mix to illustrate it, like dry ice, should be dealt with with the utmost care. Oh and here is a reference, incase anyone thinks this is a scare story. [2] —The preceding unsigned comment was added by Rockpocket (talk • contribs) 23:52, 15 April 2007 (UTC).
- That's very unfortunate for him, and it does show the capabilities of liquid nitrogen, but every job has its risks. Things malfunction and accidents do happen everywhere. --Russoc4 00:27, 16 April 2007 (UTC)
- I would guess he had suffocated from lack of oxygen. If you're breathing nitrogen gas you won't feel like you are out of breath, but you will still be dying. He could easily have gotten light headed and fallen over, knocking the LN2 dewar next to or on him. Of course I wasn't there and don't know what happened, but I don't think he froze to death. Simply put it is a substance that has many properties that we are not accustomed to, and don't encounter them in every day life. The temperature, the rapid evaporation, the potential for explosion, and more. [Mαc Δαvιs] ❖ 03:14, 16 April 2007 (UTC)
- I'll echo what's been said above. The biggest risks associated with handling liquid nitrogen aren't from the cold liquid itself. (Though I'd tend to shy away from the 'sloshing it around in your mouth' demo, as inadvertently swallowing some – hiccup! – could have very unpleasant sequalae.) For milder injuries, you're looking at contact with uninsulated surfaces (particularly metallic ones) that have been chilled with liquid nitrogen. There's a risk of frostbite, and a risk of injury if you freeze your skin to a surface. For the most serious injuries (and deaths), you're looking at the effects of hypoxia, when nitrogen dilutes or displaces oxygen from the air in a confined space. Straight oxygen starvation can do you in; there's also the risk of serious injury when you lose consciousness and fall.
- Any workplace that handles liquid nitrogen needs to provide appropriate training that addresses these risks. About a liter and a half of liquid nitrogen will boil off to form a full cubic meter of gas; LN2 should never be dispensed or transferred in a small or poorly-ventilated space. Note that many workplaces ban the transportation of liquid nitrogen containers in occupied passenger elevators for these reasons. TenOfAllTrades(talk) 03:34, 16 April 2007 (UTC)
Epoxy aroma
[edit]What makes the aroma of epoxy and polyester resin so pleasant and are the epoxies and resins with putrid smell yet perhaps with superior characteristics (heat tollerance, bonding strengh, etc.? Nebraska Bob 15:23, 15 April 2007 (UTC)
- Probably Aromatic hydrocarbon may help. These chemicals might be present in some quantity in various epoxies. Nimur 16:50, 18 April 2007 (UTC)
Alternating Current
[edit]Very simple and very basic question: What happens to the electrons in a conductor when an alternating current is passed through it? I'm interested in the displacement of electrons. (In DC, elecrons move from A to B). =Nichalp «Talk»= 15:54, 15 April 2007 (UTC)
- Well they move back and forth basically. For a typical wire the speed is very low, centimeters per second at best. —The preceding unsigned comment was added by 84.187.46.16 (talk) 16:22, 15 April 2007 (UTC).
- Heres a nice link [3] explaining it simply. After the simple explanation it gets quite contoversial. Basically the speed of the electricity is the speed of light, but the 'electrons' move quite slowly. —The preceding unsigned comment was added by 88.111.3.93 (talk) 16:28, 15 April 2007 (UTC).
- Think of it like sound moving through air. The sound moves very quickly, while the air usually moves much slower. StuRat 19:14, 15 April 2007 (UTC)
I dont think that's a good anlaogy StuRat. The compression wave and therefore the air molecules move at exactly the speed of sound in the medium. Sound is a longitudinal wave and that the above must be so is obvious. —Preceding unsigned comment added by 88.111.3.93 (talk • contribs) 19:21, 15 April 2007
- The speed of individual electrons as they jump from one atom to another is quite high, as well, but nonetheless, the average speed of all the electrons through which electrical current passes, just like the average speed of all the air molecules through which a sound wave passes, is quite low. StuRat 19:29, 15 April 2007 (UTC)
- You miss the point in that electromagnetic energy is a transverse wave phenomenon where the speed of propagation is not limited by the velocity of the individual particles 'causing' it! —Preceding unsigned comment added by 88.111.3.93 (talk • contribs) 19:35, 15 April 2007
- Neither are compression waves. Think of a long tube filled with tennis balls. You stick one in on one end; the last one pops out the other end. If you divide the length of the tube by the time needed for the last ball to pop out, you can see that the compression wave must have moved much faster than any individual ball. --Trovatore 19:38, 15 April 2007 (UTC)
- No faster than the parts of the tennis balls touching each other! —Preceding unsigned comment added by 88.111.3.93 (talk • contribs) 19:40, 15 April 2007
- I'm sorry, you're simply wrong. The compression wave does move faster than the parts of the tennis balls touching each other. --Trovatore 19:43, 15 April 2007 (UTC)
- No faster than the parts of the tennis balls touching each other! —Preceding unsigned comment added by 88.111.3.93 (talk • contribs) 19:40, 15 April 2007
- Neither are compression waves. Think of a long tube filled with tennis balls. You stick one in on one end; the last one pops out the other end. If you divide the length of the tube by the time needed for the last ball to pop out, you can see that the compression wave must have moved much faster than any individual ball. --Trovatore 19:38, 15 April 2007 (UTC)
- Even at the molecular level? Care to explain how? —Preceding unsigned comment added by 88.111.3.93 (talk • contribs) 19:45, 15 April 2007
- You're positing a difference in the propagation speed of longitudinal waves versus compression waves, or a difference between the relationship of one of those waves to some "speed" of the medium across they propagate, versus the other one. Care to explain what you mean, and what it is that "must be obvious"? (And could you please sign your posts?) —Steve Summit (talk) 19:53, 15 April 2007 (UTC)
- Thers no difference according to those links you quoted!--88.111.3.93 20:16, 15 April 2007 (UTC)
- You're positing a difference in the propagation speed of longitudinal waves versus compression waves, or a difference between the relationship of one of those waves to some "speed" of the medium across they propagate, versus the other one. Care to explain what you mean, and what it is that "must be obvious"? (And could you please sign your posts?) —Steve Summit (talk) 19:53, 15 April 2007 (UTC)
- (Edit conflict, to the anont). If you want to go down to the very lowest level, the forces are transmitted by the exchange of field quanta, which in the case of the relevant force (electromagnetic) are photons. They move at the speed of light. So if you insist on this point, the "parts of the tennis balls touching each other" are actually moving at the speed of light, and of course the compression wave doesn't travel faster than that. But that's not a normal way of understanding the phrase "the parts of the tennis balls touching each other". --Trovatore 19:53, 15 April 2007 (UTC)
- So the compression wave travels at the speed of light? Even though you insert the balls at less than c? Thats clever!.--88.111.3.93 20:08, 15 April 2007 (UTC)
- I would have thought that the compression wave would travel at the speed of sound in the medium (rubber or whatever theyre made of these days). Is that not correct? —The preceding unsigned comment was added by 88.111.93.55 (talk) 23:09, 15 April 2007 (UTC).
- Well, more or less -- the compression wave is sound, in a sense. No, it doesn't travel at the speed of light. The field quanta that transmit the force travel at the speed of light, but the wave travels much slower. I don't know where you got the idea that the compression wave has to travel at the same speed as the particles, but in any case, that idea is completely wrong. Where you do have a point, sort of, is that it can't travel faster than the force can be transmitted, and that speed is limited by the speed of the field quanta. --Trovatore 01:14, 16 April 2007 (UTC)
- I would have thought that the compression wave would travel at the speed of sound in the medium (rubber or whatever theyre made of these days). Is that not correct? —The preceding unsigned comment was added by 88.111.93.55 (talk) 23:09, 15 April 2007 (UTC).
- Or imagine a long pipe filled with water, with a faucet in (say) your bathroom, and a water heater in the basement. If you turn on or off the bathroom faucet (or if I turn on or off the shut-off valve in the basement), the flow starts or stops immediately. Yet (as everyone has experienced) it can take quite a while for the hot water to get there. —Steve Summit (talk) 19:46, 15 April 2007 (UTC)
- There's a separate issue there, though. Some of the cold water you feel in the shower was actually hot at the moment you turned on the faucet. It got cold on the way. In doing so, it heated up the pipes, and it's not until the pipes are hot that you get hot water at the shower head. --Trovatore 19:48, 15 April 2007 (UTC)
- Yeah. Is that at all pertinent to the current discussion? —The preceding unsigned comment was added by 88.110.218.16 (talk) 20:44, 15 April 2007 (UTC).
- There's a separate issue there, though. Some of the cold water you feel in the shower was actually hot at the moment you turned on the faucet. It got cold on the way. In doing so, it heated up the pipes, and it's not until the pipes are hot that you get hot water at the shower head. --Trovatore 19:48, 15 April 2007 (UTC)
I question the claim that electron drift through a conductor carrying alternating current is a transverse wave. The electrons go back and forth (longitudinally), not transversely. Make the AC frequency low enough and the waveform is indistinguishable in any short portion from slowly varying DC. If we make the AC frequency low enough, the conductor short enough and small enough in diameter, and the current high enough, the population of electrons in the conductor can move entirely out of it and be replaced in each half cycle. It is as longitudinal as water flowing through a pipe. In normal real-wporld AC applications, the power cord is long enough and large enough in cross section, and the current is low enough and the frequency high enough that the drift rate is very slow and the same old electrons drift back and forth in the power cord never going more than a few millimeters or centimeters in an average cycle. Edison 19:55, 15 April 2007 (UTC)
- I didnt say electron dirft was transverse. I said the energy wave (electromagnetic) was transverse.--88.111.3.93 20:08, 15 April 2007 (UTC)
- A good analogy for understanding the speed of electron flow would be a 10 foot length of pipe filled with ball bearings. The pipe is the wire, the ballbearings are the free electrons. If you roll a ballbearing down an empty pipe, it might maybe take a second to come out the other end, but if the pipe is full of ballbearings and you push one in one end, a different one drops out of the far end almost immediately. Hence, the speed of an individual electron can be quite slow while the fact of the presence of an electron at one end of the wire can travel to the far end at the speed of light. In A/C current, the electrons simply drift back and forth along the wire - moving very small distances indeed. SteveBaker 20:35, 15 April 2007 (UTC)
- No its not a good analogy. You are again talking longitudinal/compression waves. Electricity is a transverse wave phenomenon as every schoolboy knows. —The preceding unsigned comment was added by 88.110.218.16 (talk) 20:38, 15 April 2007 (UTC).
- So which kind of wave is a current of light? —Steve Summit (talk) 21:34, 15 April 2007 (UTC)
- As far as I know photons do not possess charge in the conventional sense and therefore their flow cannot be considered an electrical current. What you propose (ie light current) must therefore be an
enigma(oxy)moron. Light is obviously full of energy and can illuminate things. Current on the other hand is a flow of charge that cannot be stopped in the conventional sense. Light is a transverse (or orthogonal) wave. Electromagnetic energy is a transverse wave. Hope that clears that up! —The preceding unsigned comment was added by 88.110.101.245 (talk) 21:54, 15 April 2007 (UTC).- Mm... Electrical current can be stopped. -- mattb
@ 2007-04-15T22:18Z
- Mm... Electrical current can be stopped. -- mattb
- As far as I know photons do not possess charge in the conventional sense and therefore their flow cannot be considered an electrical current. What you propose (ie light current) must therefore be an
- Hmmm! But can anything stop Light current/ I think not 8-)) —The preceding unsigned comment was added by 88.109.192.18 (talk) 22:29, 15 April 2007 (UTC).
- Not mere ban hammers... Nope. -- mattb
@ 2007-04-15T23:42Z
- Not mere ban hammers... Nope. -- mattb
- You know, there's a bit of difference between a photonic electromagnetic wave and an electrical signal in a conductor. They are related phenomena, but they aren't precisely the same. The classical compression wave-like response to electric field isn't all that far off. There are actually several conduction mechanisms, but the descriptions presented are pretty accurate for a simple conductor. Perhaps that's where the schoolboy might get confused. -- mattb
@ 2007-04-15T21:23Z
- You know, there's a bit of difference between a photonic electromagnetic wave and an electrical signal in a conductor. They are related phenomena, but they aren't precisely the same. The classical compression wave-like response to electric field isn't all that far off. There are actually several conduction mechanisms, but the descriptions presented are pretty accurate for a simple conductor. Perhaps that's where the schoolboy might get confused. -- mattb
- Im not totally sure about this, but it would appear that the wave equations for travelling waves seem to be of similar form for both compresion wave and electromagnetic waves. In neither case, does the velocity of propagation relate to the particle velocity (as far as I can understand) but relates purely to the medium constants: density/stiffness of the air, or permeability/permittivity of free space.
- Maybe the relevant articles should try to explain the travelling wave phenomenon (for the case of compression waves, say) more clearly so that people like me get a deeper understanding of how it works? ie how exactly can something fast (the wave disturbnce) actually be caused by something slower (or even faster) than itself? Or am I being incredibly dense?--88.109.136.82 12:35, 16 April 2007 (UTC)
- Please do not try to discuss the details of electron movement in a conductor with classical physics. If you are interested the in details, take a look at "Feynmans Lectures on Physics, Quantum Mechanics, Propagation in a Crystal Lattice", it gives a nice introduction on electron movement in a metal. —The preceding unsigned comment was added by 84.187.32.191 (talk) 20:21, 17 April 2007 (UTC).
higher Coefficient of relationship
[edit]Is it possible for a parent, sibling, cousin or anyone to have a higher Coefficient of relationship to you than your offspring? Clem 16:52, 15 April 2007 (UTC)
- Presumably your clone would. --TotoBaggins 18:05, 15 April 2007 (UTC)
Yes. Children of completely unrelated parents (meaning no common chromosomes, in this example) should always have exactly half of each parent's chromosomes. Full siblings of unrelated parents have, on average, one half their chromosomes in common (neglecting identical twins, triplets, etc.), but this can vary from no chromosomes in common to all chromosomes in common. Thus, the chances that siblings who are not identical will have more than half their chromosomes in common is only slightly less than half (because there is some chance they have exactly half their chromosomes in common). Identical twins, triplets, etc., always have all their chromosomes in common. First cousins only have one quarter of their chromosomes in common, on average, so it would be less likely that they would have more than half their chromosomes in common, but still possible. StuRat 19:09, 15 April 2007 (UTC)
- The process that StuRat refers to does not happen at the chromosomal level. Each of your autosomal chromosomes is a patchwork of stretches from one of your parents' paternal and maternal chromosomes. See Chromosomal crossover and Meiosis. This also applies to the X-chromosome, girls inherit a patchwork of parts from their mother's maternal and paternal X-chromosomes. I'm not 100% certain about whether there may be "autosomal" parts of the Y chromosome that can cross over with corresponding parts of the X chromosome. If we assume there isn't, boys inherit the entire Y-chromosome from their father, and girls inherit the entire X-chromosome of their father. Both boys and girls inherit their mitochondrial DNA from their mother. --NorwegianBlue talk 19:41, 15 April 2007 (UTC)
Is then the Coefficient of relationship the most realistic biological means for distributing wealth and/or possessions for someone who has died without leaving a will rather than for all of their wealth and/or possessions to become the property of the state? Clem 20:00, 15 April 2007 (UTC)
- That's entirely a societal question, not one that can be answered by science. StuRat 20:19, 15 April 2007 (UTC)
- To clarify, it is a societal question StuRat but one which is intended to expose the strength/weakness or applicability/reliability of such a measure as the COR as answered below. Clem 22:22, 15 April 2007 (UTC)
- (edit conflict)
- This is a matter of legislation, not biology. Your question rests on the premise that the money should follow the genes. Where I live, there is no distinction between adoptive children and biological children in such matters, but this may vary from country to country. Such a system would easily lead to unreasonable consequences. For instance, it would imply that cousins whose fathers are identical twins should inherit an equal share of the estate of each twin. --NorwegianBlue talk 20:24, 15 April 2007 (UTC)
- Although indeed a social and legislative issue this is possibly one of the areas in which science/mathematics could help provide a fair and reasonable basis for distribution of assets in the absence of a will but mainly here I am wanting to expose the weaknesses/strengths and applicability/reliability of the measure since this it is assumed to be quit suitable for biological purposes such as organ donation. "...so you're asking me to give up a kidney since we are biologically related but yet have not for the same reason included me in your will?" Clem 22:46, 15 April 2007 (UTC)
- The reason its used in organ transplants is not as a justification for donation, but for medical reasons. The greater your COR is to the donor, the smaller the chance that the organ will be rejected. This is the same reason we wish to develop theraputic cloning, because growing a new organ with your own DNA is the equivilent of getting an organ from a clone of you (a COR = 1).
- If one could SNP map an entire human genome at a reasonable cost, one could determine roughly the proportion of DNA shared between the person who died and the potential benefactors of their will. So it could be done. But even the most strict believers in genetic determinism would find it difficult to justify why having an extra 3% of your grandfathers alleles compared to your sibling would be good cause to distribute wealth his wealth accordingly. For example, say the person with the extra 3% of the grandfather's alleles had an excess of his recessive alleles, while the person with less of grandfather's DNA in total, had a lot more dominant alleles. That would mean the person with the less DNA could be phenotypically much more like their grandfather than their sibling. Rockpocket 02:18, 16 April 2007 (UTC)
- The answer to that would of course be deferred to and ultimately determined by the Genetic Determinism Legislature and Judiciary since it is a social/legal issue! The science would simply provide a foundation or backbone, if you will, upon which to make such decisions. Clem 04:10, 16 April 2007 (UTC)
- Since many families have adopted children to link inheritance of wealth to geneotype would not be useful for all cases. David D. (Talk) 18:00, 16 April 2007 (UTC)
- The answer to that would of course be deferred to and ultimately determined by the Genetic Determinism Legislature and Judiciary since it is a social/legal issue! The science would simply provide a foundation or backbone, if you will, upon which to make such decisions. Clem 04:10, 16 April 2007 (UTC)
Nitric Acid
[edit]This is random, but I'm working on a mechanism for the oxidation of benzoin using only nitric acid, and it's annoying the hell out of me. Anyway, I'm not sure why I haven't thought of this before, but does the proton-less oxygen of nitric acid not pick up another proton, but rather it looses a proton from the OH. --Russoc4 19:01, 15 April 2007 (UTC)
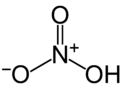
- I believe you are correct. If I remember correctly, the nitrogen and other oxygens draw the electrons away from the bond between the hydrogen and oxygen, more or less leaving the hydrogen dangling, ripe for the picking by a base. It appears to be possible to protonate nitric acid, but it takes a really really strong acid (H3 or CH5? wacky.) [4]--Bennybp 04:11, 16 April 2007 (UTC)
- Probably two important effects here. The formally positively charged nitrogen is likely important in attracting electrons from the oxygen atoms (less likely to reach out for H+ away from N+): compare the acidities of nitric acid and nitrous acid. Also, lone pairs on each of the singly-bonded oxygens are resonance-stabilized, rendering them less basic (or the protonated form of them more acidic). The nitric-acid oxidation of benzoin to benzil was promoted by adding additional acid, so presumably further protonation of HNO3 (or at least keeping nitric acid in its protonated form instead of as nitrate anion) is important to the mechanism, but last I knew the mechanism was not completely established or straightforward. DMacks 19:05, 16 April 2007 (UTC)
Passive immunity
[edit]Durring passive imunity your white blood cells still build up some ammount of active immunity?Bastard Soap 19:38, 15 April 2007 (UTC)
- Often yes. Functional immune cells may still respond to novel antigenic protein even when there are antibodies from other sources present. alteripse 19:57, 15 April 2007 (UTC)
- That's true, but just to be clear: active immunity doesn't build up in response to the passive immunity. --David Iberri (talk) 14:14, 16 April 2007 (UTC)
Wrong Article
[edit]When searching for gauss rifle, you get the article about coilguns. They are not the same. How can i make an article about gauss guns specifically? —The preceding unsigned comment was added by Dansportman (talk • contribs) 20:00, 15 April 2007 (UTC).
- Replace the redirect with your new article. 71.100.175.14 20:03, 15 April 2007 (UTC)
- The article coilgun says that a gauss gun uses electromagnets while a coilgun uses solenoids. The article railgun claims that coil guns and gauss guns are similar, but both different from a railgun. If you want to access the pages that redirect to coilgun, see this this page, however, I don't know if a change is necessary.
- It's common for us to have a redirect to something similar, and then, within that article, explain the differences. StuRat 20:15, 15 April 2007 (UTC)
Don't worry guys, it's the questioner that is wrong. What is known as a "guass gun" is exactly the same as a "coil gun." Guass/coil guns use coils and rail guns use rails. [Mαc Δαvιs] ❖ 20:34, 15 April 2007 (UTC)
Solenoid is type of electromagnet. -Yyy 06:46, 16 April 2007 (UTC)
Unknown Dianthus Image
[edit]
I took a nice photograph of a dianthus flower. Can the genus be identified so that the image can be placed in an appropriate article? Your help is much appreciated. Thegreenj 22:37, 15 April 2007 (UTC)
- Where did you take the pic? bibliomaniac15 00:19, 16 April 2007 (UTC)
- I am not sure, but I believe that it was taken a trip to Tennessee. Thegreenj 22:32, 16 April 2007 (UTC)
- It looks very similar to Dianthus plumarius, or the wild pink carnation. The picture on the dianthus article looks very similar to yours, only crappier. bibliomaniac15 00:39, 17 April 2007 (UTC)
- Can you say for sure that it is a Dianthus plumarius and not a Dianthus gratianopolitanus, which is also brought up by a web searh for the word dianthus. If you know the difference and can positively identify it one was or another, your help is still welcome... Thank you so far - Thegreenj 01:23, 17 April 2007 (UTC)
- I have identified it as a Dianthus plumarius. Thank you for your help. Thegreenj 20:05, 17 April 2007 (UTC)
Sit-ups
[edit]I always see overweight people doing sit-ups, and I wonder why, is there a reason to do them if you dont have a flat stomach? Because I know you cannot just lose fat from one area of the body —The preceding unsigned comment was added by 76.167.136.84 (talk) 22:47, 15 April 2007 (UTC).
- Situps are good for reducing lower back pain. Fat people tend to have lower back pain. I just wonder where you are seeing fat people doing excercise. I work in a hospital and I see fat people in the elevator, riding the courtesy tram, getting pushed around in wheelchairs - basically anything to avoid excercise before they see the doctor to find out how bad their hypertension, hypercholesterolemia, and diabetes is getting. --Kainaw (talk) 22:54, 15 April 2007 (UTC)
- Just because one cannot lose fat from a specific part, does not mean that one cannot gain muscle in a specific part. Situps are one way (though there are other, arguably more effective methods) of strengthening core muscles, which help us, among other things, avoid back injury, improve posture, and maintain (mechanical) balance. tucker/rekcut 00:25, 16 April 2007 (UTC)
