Venenivibrio stagnispumantis
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (September 2011) |
| Strain CP.B2 | |
|---|---|
| |
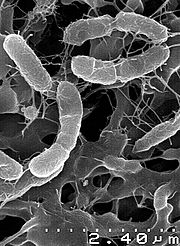
| Scanning electron microscopy image | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Aquificota |
| Order: | Aquificales |
| Family: | Hydrogenothermaceae |
| Genus: | Venenivibrio |
| Species: | V. stagnispumantis
|
| Binomial name | |
| Venenivibrio stagnispumantis Hetzer et al. 2008
| |
Venenivibrio stagnispumantis strain CP.B2 is the first microorganisms isolated from the terrestrial hot spring Champagne Pool (75 °C, pH 5.5) in Waiotapu, New Zealand.[1][2]
Morphology
[edit]The cells are motile and slightly curved rods (1.04 to 1.56 μm long and 0.33 to 0.41 μm wide).[2]
Growth conditions
[edit]The novel bacterium is an obligate chemolithotroph capable of utilizing H2 as electron donor, O2 as corresponding electron acceptor and CO2 as carbon source. Venenivibrio stagnispumantis gains metabolic energy using the "Knallgas" reaction H2 + ½ O2 → H2O. For growth either elemental sulphur (S0) or thiosulfate (S2O32−) is required. Growth is observed under thermophilic conditions between 45 °C and 75 °C (optimum 70 °C), under moderate acidophilic conditions between pH 4.8 and 5.8 (optimum pH 5.4) and under microaerophilic conditions between 1.0% and 10.0% (v/v) O2 (optimum between 4.0% and 8.0% (v/v) O2).[2]
Phylogeny
[edit]Phylogenetic analysis based on 16S rDNA sequences indicate that strain CP.B2 belongs to the order Aquificales and represents the type strain of a novel species of a new genus within the family Hydrogenothermaceae. The 16S rRNA gene sequence for strain CP.B2 is deposited in the GenBank nucleotide sequence database under accession number DQ989208. The G+C content of the genomic DNA is 29.3 mol% which is the lowest G+C content reported for a species of the order Aquificales.[2]
Tolerance to arsenic and antimony compounds
[edit]Venenivibrio stagnispumantis displays tolerance to relatively high concentrations of arsenic and antimony compounds. Cells grew in the presence of up to 8 mM arsenite (As3+), 15 mM trivalent antimony (Sb3+), and >20 mM arsenate (As5+) but growth was not observed when As3+ and As5+ were provided as the sole electron donor and acceptor pair.[2]
Nomenclature
[edit]Ve.ne.ni.vi' bri.o. (Latin: the vibrio of poison) stag.ni.spu.man' tis. (Latin: from the bubbling pool, pertaining to Champagne Pool).[2]
Culture collection
[edit]Venenivibrio stagnispumantis strain CP.B2 is deposited in the Japan Collection of Microorganisms under JCM 14244, in the American Type Culture Collection under ATCC BAA-1398 and in the German Collection of Microorganisms under DSM 18763.[2]
Culture medium
[edit]| Venenivibrio stagnispumantis culture medium | |
|---|---|
| NaOH | 0.15 g |
| KCl | 0.50 g |
| MgCl2 • 6 H2O | 1.36 g |
| MgSO4 • 7 H2O | 7.00 g |
| Na2S2O3 • 5 H2O | 2.00 g |
| CaCl2 • 2 H2O | 0.40 g |
| NH4Cl | 0.20 g |
| K2HPO4 | 0.25 g |
| Trace minerals (100x) | 10.00 ml |
| MES | 1.95 g |
| Distilled water | make up to 1000.00 ml |
The pH of the medium is adjusted to 5.5, distributed into culture vessels and autoclaved under CO2. After the inoculation the initial gas phase is replaced with H2 / CO2 / O2 (79% / 15% / 6%) and pressurized to 170 kPa.[2]
| Trace mineral solution (100x) | |
|---|---|
| Na2-EDTA • 2 H2O | 500.00 mg |
| CoCl2 • 6 H2O | 150.00 mg |
| MnCl2 • 4 H2O | 100.00 mg |
| FeSO4 • 7 H2O | 100.00 mg |
| ZnCl2 | 100.00 mg |
| AlCl3 • 6 H2O | 40.00 mg |
| Na2WO4 • 2 H2O | 30.00 mg |
| CuCl2 • 2 H2O | 20.00 mg |
| Ni2SO4 • 6 H2O | 20.00 mg |
| Na2SeO3 | 10.00 mg |
| H3BO3 | 10.00 mg |
| Na2MoO4 • 2 H2O | 10.00 mg |
| Distilled water | make up to 1000.00 ml |
The pH of the solution is adjusted to 3.0 with HCl.[2]
References
[edit]- ^ Hetzer, Adrian; Morgan, Hugh W.; McDonald, Ian R.; Daughney, Christopher J. (2007). "Microbial life in Champagne Pool, a geothermal spring in Waiotapu, New Zealand". Extremophiles. 11 (4): 605–14. doi:10.1007/s00792-007-0073-2. PMID 17426919. S2CID 24239907.
- ^ a b c d e f g h i Hetzer, A.; McDonald, I. R.; Morgan, H. W. (2008). "Venenivibrio stagnispumantis gen. nov., sp. nov., a thermophilic hydrogen-oxidizing bacterium isolated from Champagne Pool, Waiotapu, New Zealand". International Journal of Systematic and Evolutionary Microbiology. 58 (2): 398–403. doi:10.1099/ijs.0.64842-0. PMID 18218938.
External links
[edit] Media related to Venenivibrio stagnispumantis at Wikimedia Commons
Media related to Venenivibrio stagnispumantis at Wikimedia Commons- Type strain of Venenivibrio stagnispumantis at BacDive - the Bacterial Diversity Metadatabase
