User:WingmanJ/sandbox

Spiroligomers (also known as bis-peptides) are synthetic oligomers made by coupling pairs of bis-amino acids into a fused ring system. [1] Spiroligomers are rich in stereochemistry and functionality because of the variety of bis-amino acids that are capable of being incorporated during synthesis. [2] Due to the rigidity of the fused ring system,[3] the three-dimensional shape of a spiroligomer – as well as the display of any functional groups – can be predicted, allowing for molecular modeling and dynamics.
Synthesis
[edit]
Spiroligomers are synthesized in a step-wise approach by adding a single bis-amino acid at each stage of the synthesis. This stepwise elongation allows for complete control of the stereochemistry, as any bis-amino acid can be incorporated to allow for elongation; or any mono-amino acid can be added to terminate a chain. This can be accomplished using either solution-phase or solid-phase reactions.[4] The original synthesis of spiroligomers allowed for functionalization on the ends of the oligomers, but it did not allow for the incorporation of functionality on the interior diketopiperazine (DKP) nitrogens.[5] Much work has been done to allow for the functionalization of the entire Spiroligomer, as opposed to just the ends.[2] By exploiting a neighboring group effect, [6] spiroligomers can be synthesized with a variety of functional groups along the length of the molecule.
Structure
[edit]Spiroligomers can be synthesized in any direction, and between any pair of bis-amino acids. Spiroligomer diketopiperazines can be created between either end of a bis-amino acid. Spiroligomers are known to be conformationally rigid, due to the fused-ring backbone. [3]
Chemical Characteristics
[edit]Spiroligomers are peptidomimetics. Completely Resistant to proteases. Not likely to raise an immune response.
Uses
[edit]Spiroligomers have been utilized for a variety of applications which include catalysis, protein binding, metal-binding, molecular scaffolds, and charge-transfer studies, et al.
Catalysis
[edit]Two unique types of spiroligomer catalysts (spiroligozymes) have been developed, an esterase mimic and a Claisen catalyst.
Transesterification
[edit]The first spiroligomer catalyst was an esterase-mimic, which catalyzed the transfer of a trifluoroacetate group. [7]

Aromatic Claisen Rearrangement
[edit]The second spiroligomer catalyst accelerated an aromatic Claisen rearrangement with a catalytic dyad similar to that found in Ketosteroid Isomerase.[8]

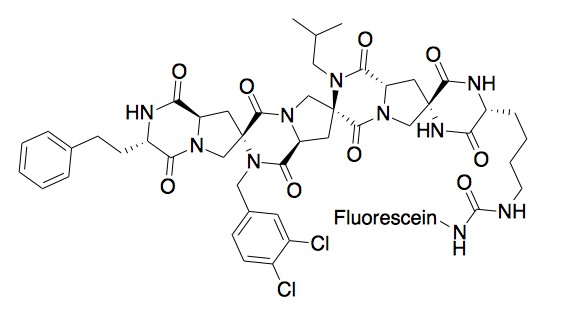
Protein Binding
[edit]A spiroligomer was designed to mimic P53 and bind HDM2. The molecule enters cells through passive diffusion, and interestingly, this mimic was shown to stabilize HDM2 in cell culture. [9]
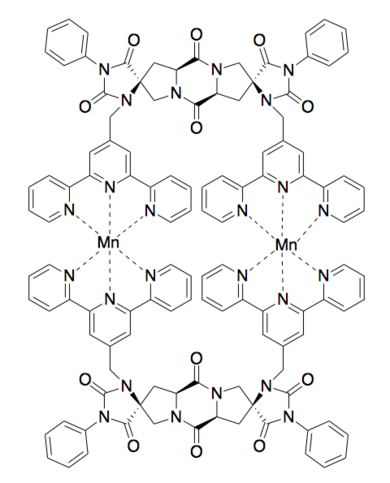
Metal Binding
[edit]Binuclear Metal Binding [10]
Molecular Scaffolds
[edit]Rods used for distance measuring with spin probes [3]
Electron Transfer
[edit]Donor-Bridge-Acceptor [11]
References
[edit]- ^ Christian E. Schafmeister, Zachary Z. Brown, and Sharad Gupta “Shape-Programmable Macromolecules” Accounts of Chemical Research, (2008), 41(10), 1387-1398 doi:10.1021/ar700283y
- ^ a b Zachary Z. Brown and Christian E. Schafmeister, “Synthesis of Hexa- and Pentasubstituted Diketopiperazines from Sterically Hindered Amino Acids” Organic Letters, (2010), 12(7), 1436-1439 doi:10.1021/ol100048g
- ^ a b c Gregory H. Bird, Soraya Pornsuwan, Sunil Saxena, and Christian E. Schafmeister, “Distance Distributions of End-Labeled Curved Bispeptide Oligomers by Electron Spin Resonanace” ACSNano (2008), 2(9), 1857-1864 doi:10.1021/nn800327g
- ^ Zachary Z. Brown, Jennifer Alleva, and Christian E. Schafmeister, “Solid-Phase Synthesis of Functionalized Bis-Peptides” Biopolymers, (2011), 96(5), 578-585 doi:10.1002/bip.21591
- ^ Christopher G. Levins and Christian E. Schafmeister, “The Synthesis of Functionalized Nanoscale Molecular Rods of Defined Length” Journal of the American Chemical Society, (2003), 125(16), 4702-4703 doi:10.1021/ja0293958
- ^ Zachary Z. Brown and Christian E. Schafmeister, “Exploiting an Inherent Neighboring Group Effect of alpha-Amino Acids to Synthesize Extremely Hindered Dipeptides” Journal of the American Chemical Society, (2008), 130(44), 14382-14383 doi:10.1021/ja806063k
- ^ Mahboubeh Kheirabadi and Christian Schafmeister, et al, “Spiroligozymes for Transesterifications: Design and Relationship of Structure to Activity” Journal of the American Chemical Society, (2012), 134, 18345-18353 doi:10.1021/ja3069648
- ^ Matthew F. L. Parker and Christian E. Schafmesiter, et al, “Acceleration of an Aromatic Claisen Rearrangement via a Designed Spiroligozyme Catalyst that Mimics the Ketosteroid Isomerase Catalytic Dyad” Journal of the American Chemical Society, (2014), 136(10), 3817-3827 doi:10.1021/ja409214c
- ^ Zachary Z. Brown, Kavitha Akula, and Christian Schafmeister, et al, “A Spiroligomer alpha-Helix Mimic That Binds HDM2, Penetrates Human Cells, and Stabilizes HDM2 in Cell Culture” PLOSOne (2012), 7(10), doi:10.1371/journal.pone.0045948
- ^ Shivaiah Vaddypally, Chongsong Xu, Senzhi Zhao, Yanfeng Fan, Christian E. Schafmeister and Michael J. Zdilla “Architectural Spiroligomers Designed for Binuclear Metal Complex Templating” Inorganic Chemistry, (2013), 52, 6457-6463 doi:10.1021/ic4003498
- ^ Subhasis Chakrabarti, Matthew F. L. Parker, Christopher W. Morgan, Christian E. Schafmeister, and David H. Waldeck, “Experimental Evidence for Water Mediated Electron Transfer Through Bis-Amino Acid Donor-Bridge-Acceptor Oligomers” Journal of the American Chemical Society, (2009), 131(6), 2044-2045 doi:10.1021/ja8079324

