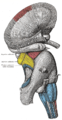
User:WatchAndObserve/Hippocampus
| WatchAndObserve/Hippocampus | |
|---|---|
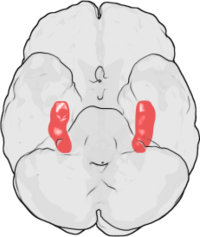 The hippocampus is structurally located inside the medial temporal lobe of the brain. (In this illustration of the underside of the brain, the frontal lobe of the brain is at the top, while the occipital lobe is at the bottom.) | |
 The location of one of the hippocampi is indicated by the crosshairs | |
| Anatomical terms of neuroanatomy |
The hippocampus is a part of the brain located in the medial temporal lobe (humans and other mammals have two hippocampi, one in each side of the brain). It forms a part of the limbic system and plays a part in memory and spatial navigation. The name derives from its curved shape in coronal sections of the brain, which resembles a seahorse (Greek: hippos = horse, kampi = curve).
In Alzheimer's disease, the hippocampus is one of the first regions of the brain to suffer damage; memory problems and disorientation appear among the first symptoms. Damage to the hippocampus can also result from oxygen starvation (anoxia) and encephalitis.
Role in general memory
[edit]Psychologists and neuroscientists dispute the precise role of the hippocampus, but, in general, agree that it has an essential role in the formation of new memories about experienced events (episodic or autobiographical memory). Some researchers prefer to consider the hippocampus as part of a larger medial temporal lobe memory system responsible for general declarative memory (memories that can be explicitly verbalized — these would include, for example, memory for facts in addition to episodic memory).
Some evidence supports the idea that, although these forms of memory often last a lifetime, the hippocampus ceases to play a crucial role in the retention of the memory after a period of consolidation. Damage to the hippocampus usually results in profound difficulties in forming new memories (anterograde amnesia), and normally also affects access to memories prior to the damage (retrograde amnesia). Although the retrograde effect normally extends some years prior to the brain damage, in some cases older memories remain - this sparing of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain. However, experimentation has difficulties in testing the sparing of older memories; and, in some cases of retrograde amnesia, the sparing appears to affect memories formed decades before the damage to the hippocampus occurred, so its role in maintaining these older memories remains controversial.
Damage to the hippocampus does not affect some aspects of memory, such as the ability to learn new skills (playing a musical instrument, for example), suggesting that such abilities depend on a different type of memory (procedural memory) and different brain regions. And there is evidence to suggest that patient HM (who had his medial temporal lobes removed bilaterally as a treatment for epilepsy[1]) can form new semantic memories.[2]
Role in spatial memory and navigation
[edit]Some evidence implicates the hippocampus in storing and processing spatial information. Studies in rats have shown that neurons in the hippocampus have spatial firing fields. These cells are called place cells. Some cells fire when the animal finds itself in a particular location, regardless of direction of travel, while most are at least partially sensitive to head direction and direction of travel. In rats, some cells, termed context-dependent cells, may alter their firing depending on the animal's past (retrospective) or expected future (prospective). Different cells fire at different locations, so that, by looking at the firing of the cells alone, it becomes possible to tell where the animal is. Place cells have now been seen in humans involved in finding their way around in a virtual reality town. The findings resulted from research with individuals that had electrodes implanted in their brains as a diagnostic part of surgical treatment for serious epilepsy.[3]
The discovery of place cells led to the idea that the hippocampus might act as a cognitive map — a neural representation of the layout of the environment. Recent evidence has cast doubt on this perspective, indicating that the hippocampus might be crucial for more fundamental processes within navigation. Regardless, studies with animals have shown that an intact hippocampus is required for simple spatial memory tasks (for instance, finding the way back to a hidden goal).
Without a fully-functional hippocampus, humans may not successfully remember where they have been and how to get where they are going. Researchers believe that the hippocampus plays a particularly important role in finding shortcuts and new routes between familiar places. Some people exhibit more skill at this sort of navigation than do others, and brain imaging shows that these individuals have more active hippocampi when navigating.
London's taxi drivers must learn a large number of places — and know the most direct routes between them (they have to pass a strict test, The Knowledge, before being licensed to drive the famous black cabs). A study at University College London by Maguire, et al (2000) showed that part of the hippocampus is larger in taxi drivers than in the general public, and that more experienced drivers have bigger hippocampi.[4] Whether having a bigger hippocampus helps an individual to become a cab driver or finding shortcuts for a living makes an individual's hippocampus grow is yet to be elucidated. However, in that study Maguire, et al examined the correlation between size of the grey matter and length of time that had been spent as a taxi driver, and found that the longer an individual had spent as a taxi driver, the larger the volume of the right hippocampus.[4] This finding suggested to the authors that increases in size with use over time.[4]
A study on rats at Indiana University suggested that the sexual dimorphism in the hippocampus morphology is tied to a sexual dimorphism in repeated maze performance.[citation needed] Males seem to be better at contexualizing their whereabouts because they have more hippocampus to work with.
History
[edit]
The anatomist Giulio Cesare Aranzi (circa 1564) first used the term hippocampus to describe the cerebral organ because of its visual resemblance to a seahorse. This organ was initially connected with the sense of smell, rather than with its known function in memory acquisition. The Russian Vladimir Bekhterev noted the role of the hippocampus in memory around 1900, based on observations of a patient with profound memory disturbances. However, for many years, the conventional view of the hippocampus was that, like the rest of the limbic system, it was responsible for emotion.
The importance of the hippocampus in memory was brought to the attention of researchers by patient HM. HM suffered from a number of anterograde and temporally-graded retrograde memory impairments (such impairments are the subject of the movie Memento) following the bilateral removal of various medial-temporal lobe structures (including bilateral ablation of his hippocampi) to relieve frequent epileptic seizures. Of particular importance is that HM was still able to learn procedural tasks (which are associated with the Striatum) and had an above-average IQ. HM demonstrated a striking single-dissociation between intelligence and declarative memory. The relative size of the hippocampal formation in relation with the total volume of the brain is often conserved in most of the mammalian species. Nevertheless, it has been found that these areas are relatively hypotrophic in cetaceans.
Anatomy
[edit]
Although there is a lack of consensus relating to terms describing the hippocampus and the adjacent cerebral cortex, the term hippocampal formation generally applies to the dentate gyrus, the Cornu Ammonis fields CA1-CA3 (and CA4, frequently called the hilus and considered part of the dentate gyrus), and the subiculum. The CA1, CA2 and CA3 fields make up the hippocampus proper.
Information flow through the hippocampus proceeds from the dentate gyrus to CA3 to CA1 to the subiculum, with additional input information at each stage and outputs at each of the two final stages. CA2 represents only a very small portion of the hippocampus and its presence is often ignored in accounts of hippocampal function, though it is notable that this small region seems unusually resistant to conditions that usually cause large amounts of cellular damage, such as epilepsy.
The perforant path, which brings information primarily from entorhinal cortex (but also perirhinal cortex, among others), is generally considered the main source of input to the hippocampus. Layer II of entorhinal cortex (EC) brings input to the dentate gyrus and field CA3, while EC layer III brings input to field CA1 and the subiculum. The main output pathways of the hippocampus are the cingulum bundle and the fimbria/fornix, which arise from field CA1 and the subiculum.

Perforant path input from EC layer II enters the dentate gyrus and is relayed to region CA3 (and to mossy cells, located in the hilus of the dentate gyrus, which then send information to distant portions of the dentate gyrus where the cycle is repeated). Region CA3 combines this input with signals from EC layer II and sends extensive connections within the region and also sends connections to region CA1 through a set of fibers called the Schaffer collaterals. Region CA1 receives input from the CA3 subfield, EC layer III and the nucleus reuniens of the thalamus (which project only to the terminal apical dendritic tufts in the stratum lacunosum-moleculare). In turn, CA1 projects to the subiculum as well as sending information along the aforementioned output paths of the hippocampus. The subiculum is the final stage in the pathway, combining information from the CA1 projection and EC layer III to also send information along the output pathways of the hippocampus.
The hippocampus also receives a number of subcortical inputs. In Macaca fascicularis, these inputs include the amygdala (specifically the anterior amygdaloid area, the basolateral nucleus, and the periamygdaloid cortex), the medial septum and the diagonal band of Broca, the claustrum, the substantia innominata and the basal nucleus of Meynert, the thalamus (including the anterior nuclear complex, the laterodorsal nucleus, the paraventricular and parataenial nuclei, the nucleus reuniens, and the nucleus centralis medialis), the lateral preoptic and lateral hypothalamic areas, the supramammillary and retromammillary regions, the ventral tegmental area, the tegmental reticular fields, the raphe nuclei (the nucleus centralis superior and the dorsal raphe nucleus), the nucleus reticularis tegementi pontis, the central gray, the dorsal tegmental nucleus, and the locus coeruleus.
It is widely accepted that each of these regions has a unique functional role in the information processing of the hippocampus, but to date the specific contribution of each region is poorly understood.
References
[edit]- ^ Scoville, WB (1957). "Loss of Recent Memory After Bilateral Hippocampal Lesions". Journal of Neurology, Neurosurgery and Psychiatry. 20 (1): 11–21. doi:10.1136/jnnp.20.1.11. PMC 497229. PMID 13406589. PMID. Retrieved 2007-02-11.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ O'Kane, Gail; Kensinger, Elizabeth A.; Corkin, Suzanne (2004). "Evidence for semantic learning in profound amnesia: An investigation with patient H.M." Hippocampus. 14 (4): 417–425. doi:10.1002/hipo.20005. PMID 15224979. Retrieved 2007-02-11.
- ^ Ekstrom, Arne D.; Kahana, Michael J.; Caplan, Jeremy B.; Fields, Tony A.; Isham, Eve A.; Newman, Ehren L.; Fried, Itzhak (2003). "Cellular networks underlying human spatial navigation" (PDF). Nature. 425 (6954): 184–188. doi:10.1038/nature01964. PMID 12968182. Retrieved 2007-02-11.
- ^ a b c Maguire, Eleanor A.; Gadian, David G.; Johnsrude, Ingrid S.; Good, Catriona D.; Ashburner, John; Frackowiak, Richard S. J.; Frith, Christopher D. (2000). "Navigation-related structural change in the hippocampi of taxi drivers". Proceedings of the National Academy of Sciences of the United States of America. 97 (8). National Academy of Sciences: 4398–4403. doi:10.1073/pnas.070039597. PMC 18253. PMID 10716738.
Additional images
[edit]-
Superficial dissection of brain-stem. Lateral view.
-
Coronal section of brain immediately in front of pons.
-
Posterior and inferior cornua of left lateral ventricle exposed from the side.
-
Inferior and posterior cornua, viewed from above.
-
The fornix and corpus callosum from below.