User:Tingstephen/sandbox
Mechanism
[edit]Part 1: PSPP Formation from FPP
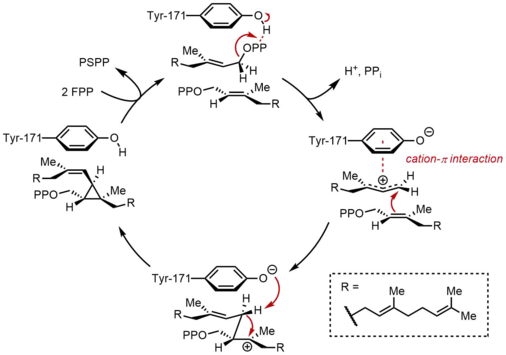
[edit]The reaction begins with the ionization of FPP to generate an allylic carbocation. A tyrosine residue (Tyr-171) plays a critical role in this step by serving as a proton donor to facilitate abstraction of pyrophosphate. Moreover, the resulting phenolate anion can stabilize the resulting carbocation through cation-π interactions, which would be particularly strong due to the highly electron-rich nature of the phenolate anion. The allylic cation generated is then attacked by the olefin of a second molecule of FPP, affording a tertiary carbocation. The phenolate anion generated previously then serves as a base to abstract a proton from this adduct to form a cyclopropane-containing compound, presqualene pyrophosphate (PSPP). The importance of a tyrosine residue in this process was demonstrated by mutagenesis studies with rSQS (Gu), and by the fact that Tyr-171 is conserved in all known SQSs (and PHSs) (Tansey, Shechter). In rSQS, Tyr-171 was converted to aromatic residues Phe and Trp, as well as hydroxyl-containing residue Ser. None of these mutants were able to convert FPP to PSPP or squalene, demonstrating that aromatic rings or alcohols alone are insufficient for converting FPP to PSPP.
Part 2: Squalene formation from PSPP
[edit]Following the formation of PSPP, squalene is formed by a series of carbocation rearrangements.[1][2] The process begins with ionization of pyrophosphate, giving a cyclopropylcarbinyl cation. The cation rearranges by a 1,2-migration of a cyclopropane C–C bond to the carbocation, forming the bond shown in blue to give a cyclobutyl carbocation. Subsequently, a second 1,2-migration occurs to form another cyclopropylcarbinyl cation, with the cation resting on a tertiary carbon. This resulting carbocation is then ring-opened by a hydride delivered by NADPH, giving squalene as the final product.
While cyclopropylcarbinyl-cyclopropylcarbinyl rearrangements can proceed through discrete cyclobutyl cation intermediates, the supposed cyclobutyl cation could not be trapped in model studies. Thus, the cyclobutyl cation may actually be a transition state between the two cyclopropylcarbinyl cations, rather than a discrete intermediate. The stereochemistry of the intermediates and the olefin geometry in the final product is dictated by the suprafacial nature of the 1,2-shifts and stereoelectronic requirements. While other mechanisms have been proposed, the mechanism shown below is supported by isolation of rillingol, which is the alcohol formed from trapping the second cyclopropylcarbinyl radical with water.
Regulation
[edit]- ^ Blagg, Brian S. J.; Jarstfer, Michael B.; Rogers, Daniel H.; Poulter, C. Dale (2002-07-04). "Recombinant Squalene Synthase. A Mechanism for the Rearrangement of Presqualene Diphosphate to Squalene". Journal of the American Chemical Society. 124 (30): 8846–8853. doi:10.1021/ja020411a.
- ^ Jarstfer, Michael B.; Blagg, Brian S. J.; Rogers, Daniel H.; Poulter, C. Dale (1996-12-25). "Biosynthesis of Squalene. Evidence for a Tertiary Cyclopropylcarbinyl Cationic Intermediate in the Rearrangement of Presqualene Diphosphate to Squalene". Journal of the American Chemical Society. 118 (51): 13089–13090. doi:10.1021/ja963308s.