User:Susan Schneegans/sandbox/Life sciences in the United States
The USA carries out 46% of global research and development (R&D) in life sciences, making it the world leader. In 2014, six US biopharmaceutical companies figured in the global Top 50 (1) for the volume of expenditure on R&D. The year before, US pharmaceutical companies had spent $ 40 billion on R&D inside the USA and nearly another $ 11 billion on R&D abroad.[1]
Federal expenditure on life sciences
[edit]Life sciences accounted for 51% of federal research expenditure in 2011. However, this expenditure has failed to keep pace with inflation, in spite of the emerging needs of an ageing population.
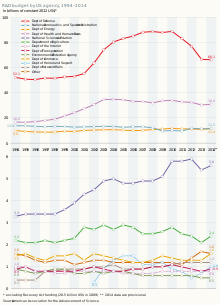
The National Institutes of Health (NIH) are considered the government's flagship biomedical research funding organization. Since 2004, NIH funding has remained flat and is even decreasing when inflation is taken into consideration. It is estimated that the NIH's 2016 budget will increase by 3.3% to $31.3 billion, $1 billion more than in the FY2015 budget. However, this is lower than in 2003-2005 when the NIH budget peaked at circa $35 billion a year.
Government efforts to increase allocations to research between 2013 and 2016 were often thwarted by th congressional austerity drive, with Congress withholding approval of the federal government's budget several times. Over this period, the executive’s priorities were, thus, taken forward largely thanks to collaboration between the government, industry and the non-profit sector. This was particularly true for the health sector which, like climate change, was a priority for the Obama administration.[1]
Better therapies in less time at a lower cost
[edit]A key policy objective of the Obama administration was to develop more targeted therapies while reducing the time and cost of drug development. Developing a new drug takes well over a decade and has a failure rate of more than 95%. The most expensive failures happen in late phase clinical trials. It is thus vital to pinpoint the right biological targets (genes, proteins and other molecules) early in the process, so as to design more rational drugs and better tailored therapies.[1]
The 21st Century Cures Act was signed into law on 13 December 2016, a year after the release of the UNESCO Science Report. The report had predicted that, ‘were the bill to pass into law, it would alter the way in which clinical trials are conducted by allowing new and adaptive trial designs that factor in personalized parameters, such as biomarkers and genetics. This provision has proven controversial, with doctors cautioning that overreliance on biomarkers as a measure of efficacy can be misleading, as they may not always reflect improved patient outcomes'.[1]
Another government scheme hopes to increase the number of new diagnostics and therapies for patients, while reducing the time and cost of developing these. At the launch of the Accelerating Medicines Partnership in February 2014, NIH director Francis S. Collins stated that 'Currently, we are investing too much money and time in avenues that don't pan out, while patients and their families wait'. Over the five years to 2019, this public−private partnership is developing up to five pilot projects for three common but difficult-to-treat diseases: Alzheimer’s disease, type 2 (adult onset) diabetes and the autoimmune disorders of rheumatoid arthritis and lupus.[1]

The partnership involves the National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as well as 10 major biopharmaceutical companies and several non-profit organizations like the Alzheimer's Association. The industrial partners are Abbvie (USA), Biogen (USA), Bristol-Myers Squibb (USA), GlaxoSmithKline (UK), Johnson & Johnson (USA), Lilly (USA), Merck (USA), Pfizer (USA), Sanofi (France) and Takeda (Japan).[1]
Laboratories share samples, such as blood or brain tissue from deceased patients, to identify biomarkers. They also participate in NIH clinical trials. One critical component is that industry partners have agreed to make the data and analyses arising from the partnership accessible to the broad biomedical community. They will not use any discoveries to develop their own drug until these findings have been made public.[1]
In April 2013, the government announced another public−private partnership, this time to implement its Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. The goal of this project is to leverage genetic, optical and imaging technologies to map individual neurons and complex circuits in the brain, eventually leading to a more complete understanding of this organ’s structure and function. By 2015, the BRAIN Initiative had ‘obtained commitments of over US$ 300 million in resources from federal agencies (National Institutes of Health, Food and Drug Administration, National Science Foundation, etc.), industry (National Photonics Initiative, General Electric, Google, GlaxoSmithKline, etc.) and philanthropy (foundations and universities)’.[1]
The Precision Medicine Initiative has been another government priority. Defined as delivering the right treatment to the right patient at the right time, precision medicine tailors treatments to patients based on their unique physiology, biochemistry and genetics. In his 2016 budget request, the president asked for US$ 215 million to be shared by the NIH, National Cancer Institute and FDA to fund the Precision Medicine Initiative.[1]
Between 2005 and 2010, pharmaceutical and biopharmaceutical companies increased their own investment in precision medicine by roughly 75% and a further increase of 53% is projected by 2015. Between 12% and 50% of the products in their drug development pipelines are related to personalized medicine.[1]
Trends in prescription drug prices
[edit]One policy concern for the Obama administration has been the steep rise in the price of prescription drugs, in a country where these prices are largely unregulated. From January 2008 to December 2014, the price of commonly used branded drugs increased by a little over 127%, even as the price of commonly prescribed generic drugs decreased by almost 63%. .[1]
In 2014, spending on prescription drugs hit $374 billion. This increase in spending was fuelled by the costly new drugs on the market for treating hepatitis C ($ 11 billion), rather than by the millions of newly insured Americans under the Patient Protection and Affordable Care Act of 2010 ($ 1 billion). About 31% of this spending went on specialty drug therapies to treat inflammatory conditions, multiple sclerosis, oncology, hepatitis C and HIV, etc., and 6.4% on traditional therapies to treat diabetes, high cholesterol, pain, high blood pressure and heart disease, asthma, depression and so on’.[1]
Fuelling the 'astronomic' rise in consumer prices for prescription drugs has been a new trend in the USA, the acquisition of pharmaceuticals through licensing, purchase, a merger or acquisition. In the first half of 2014, the value of mergers and acquisitions by pharmaceutical companies totalled US$ 317.4 billion and, in the first quarter of 2015, the drug industry accounted for a little more than 45% of all US mergers and acquisitions. Several pharmaceutical companies have made strategic mergers in recent years to relocate their headquarters overseas to order to gain a tax advantage. Pfizer’s own attempt to take over the British pharmaceutical company Astrazeneca aborted in 2014, after Pfizer admitted plans to cut research spending in the combined company.[1]
Trends in venture capital investment in life sciences
[edit]The National Venture Capital Association reported that, ‘in 2014, venture capital investment in the life sciences was at its highest level since 2008: in biotechnology, $6.0 billion was invested in 470 deals and, in life sciences overall, $ 8.6 billion in 789 deals (including biotechnology and medical devices). Two-thirds (68%) of the investment in biotechnology went to first-time/early-stage development deals and the remainder to the expansion stage of development (14%), seed-stage companies (11%) and late-stage companies (7%).[1]
However, it was the software industry which invested in the greatest number of deals overall: 1 799, for an investment of $ 19.8 billion. Second came internet-specific companies, garnering US$ 11.9 billion in investment through 1 005 deals. Many of these companies are based in the State of California, which alone concentrates 28% of US research.[1]
Total investment in venture capital amounted to US$ 48.3 billion in 2014, for 4 356 deals. This represented ‘an increase of 61% in dollars and a 4% increase in deals over the prior year,’ reported the National Venture Capital Association.[1]
Bringing down the cost to consumers
[edit]The Biologics Price Competition and Innovation Act was signed into law in March 2010 to encourage the development of generic drug competition as a cost containment measure for high-priced pharmaceuticals. Part of the government’s signatory Patient Protection and Affordable Care Act, it has created a pathway for fast-track licensure for biological products that are shown to be ‘biosimilar’ to, or ‘interchangeable’ with, an approved biological product. One inspiration for the act was that the patents for many biologic drugs will expire in the next decade.[1]
Although the act was passed in 2010, the first biosimilar was only approved in the USA by the FDA in 2015: Zarxio, made by Sandoz. Zarxio is a biosimilar of the cancer drug Neupogen, which boosts the patient’s white blood cells to ward off infection. In September 2015, a US court ruled that the Neupogen brand manufacturer Amgen could not block Zarxio from being sold in the USA. Neupogen costs about US$ 3 000 per chemotherapy cycle; Zarxio hit the US market on 3 September 2015 at a 15% discount.[1]
In Europe, the same drug had been approved as early as 2008 and has been safely marketed there ever since. The lag in development of an approval pathway in the USA has been criticized for impeding access to biological therapies.[1]
The true cost savings from the use of biosimilars is difficult to assess. A 2014 study by the Rand Institute estimates a range of US$ 13–66 billion in savings over 2014–2024, depending upon the level of competition and FDA regulatory approval patterns.[1]
Unlike generics, biosimilars cannot be approved on the basis of minimal and inexpensive tests to prove bioequivalence. Since biological drugs are complex, heterogeneous products derived from living cells, they can only be shown to be highly similar to the appropriate reference product and therefore require demonstration that there are no clinically meaningful differences in safety and efficacy’. The extent to which clinical trials are required will largely determine the cost of development.[1]
Source
[edit]![]() This article incorporates text from a free content work. Licensed under CC-BY-SA IGO 3.0. Text taken from UNESCO Science Report: towards 2030, UNESCO.
This article incorporates text from a free content work. Licensed under CC-BY-SA IGO 3.0. Text taken from UNESCO Science Report: towards 2030, UNESCO.
