User:Smokefoot/sandbox5



A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5, acylium ions RCO+ cations.[1] Until the early 1970s, carbocations were called carbonium ions.[2] In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon:
- three in the carbenium ions
- five in the carbonium ions.
This nomenclature was proposed by G. A. Olah.[3] Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds.
Since the late 1990s, most textbooks have stopped using the term carbonium ion for the classical three-coordinate carbocation.
Divergent definitions
[edit]Some authors more narrowly defined the term 'carbonium ion' as formally protonated or alkylated alkanes (CR+
5, where R is H or alkyl), to the exclusion of non-classical carbocations like the 2-norbornyl cation.[4]
Some university-level textbooks continue to use the term carbocation as if it were synonymous with carbenium ion,[5][6] or discuss carbocations with only a fleeting reference to the older terminology of carbonium ions[7] or carbenium and carbonium ions.[8] One textbook retains the older name of carbonium ion for carbenium ion to this day, and uses the phrase hypervalent carbonium ion for CH+
5.[9]
History
[edit]The history of carbocations dates back to 1891 when G. Merling[10] reported that he added bromine to tropylidene (cycloheptatriene) and then heated the product to obtain a crystalline, water-soluble material, C
7H
7Br. He did not suggest a structure for it; however, Doering and Knox[11] convincingly showed that it was tropylium (cycloheptatrienylium) bromide. This ion is predicted to be aromatic by Hückel's rule.
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol gives deep-yellow solutions in concentrated sulfuric acid. Triphenylmethyl chloride similarly formed orange complexes with aluminium and tin chlorides. In 1902, Adolf von Baeyer recognized the salt-like character of the compounds formed. The trityl carbocation (shown below) is a stable carbocationic system that has been used as homogeneous organocatalyst in organic synthesis,[12] for example in the form of trityl hexafluorophosphate.[13]
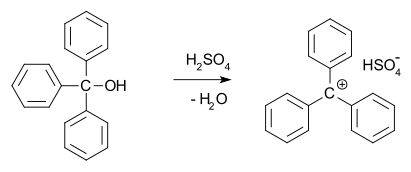
He dubbed the relationship between color and salt formation halochromy, of which malachite green is a prime example.
The idea that carbocations are reactive intermediates was proposed by Julius Stieglitz in 1899,[14] was further developed by Hans Meerwein in his 1922 study[15][16] of the Wagner–Meerwein rearrangement. Carbocations were also found to be involved in the SN1 reaction, the E1 reaction, and in rearrangement reactions such as the Whitmore 1,2 shift. The chemical establishment was reluctant to accept the notion of a carbocation and for a long time the Journal of the American Chemical Society refused articles that mentioned them.
NMR detection
[edit]The first NMR spectrum of a carbocation in solution was published by Doering et al.[17] in 1958. It was the heptamethylbenzenium ion, made by treating hexamethylbenzene with methyl chloride and aluminium chloride. The stable 7-norbornadienyl cation was prepared by Story et al. in 1960[18] by reacting norbornadienyl chloride with silver tetrafluoroborate in sulfur dioxide at −80 °C. The NMR spectrum established that it was non-classically bridged (the first stable non-classical ion observed).
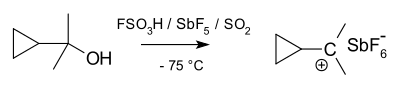
In 1962, Olah directly observed the tert-butyl carbocation by nuclear magnetic resonance as a stable species on dissolving tert-butyl fluoride in magic acid. The NMR of the norbornyl cation was first reported by Schleyer et al.[19] and it was shown to undergo proton-scrambling over a barrier by Saunders et al.[20]
Non-classical ions
[edit]Some carbocations such as the 2-norbornyl cation exhibit more or less symmetrical three-center two-electron bonding. Such structures, referred to as non-classical carbocations, involve the delocalization of the bonds involved in the σ-framework of the molecule, resulting in C–C and C–H bonds of fractional bond order.[21][22] This delocalization results in additional stabilization of the cation. For instance, depicted as a classical carbenium ion, 2-norbornyl cation appears to be a secondary carbocation. However, it is more stable than a typical "secondary" carbocation, being roughly as stable as a tertiary carbocation like t-butyl cation, according to hydride ion affinity.
Specific carbocations
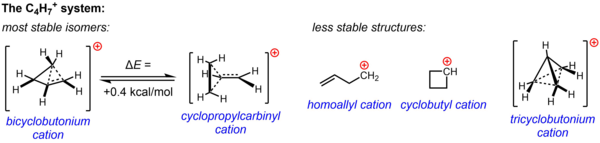
[edit]A non-classical structure for C
4H+
7 is supported by substantial experimental evidence from solvolysis experiments and NMR studies. One or both of two structures, the cyclopropylcarbinyl cation and the bicyclobutonium cation, were invoked to account for the observed reactivity in various experiments, while the NMR spectrum consists of two 13C NMR signals, even at −132 °C. Computations indicate that the bicyclobutonium structure is 0.4 kcal/mol more stable than the cyclopropylcarbinyl structure. In the solution phase (SbF5·SO2ClF·SO2F2, with SbF–
6 as the counterion), the bicyclobutonium structure predominates over the cyclopropylcarbinyl structure in a 84:16 ratio at −61 °C.[citation needed]
conducted in non-nucleophilic media
Three other possible structures, two classical structures (the homoallyl cation and cyclobutyl cation) and a more highly delocalized non-classical structure (the tricyclobutonium ion), are now known to be less stable isomers (or merely a transition state rather than an energy minimum in the case of the cyclobutyl cation).[23]
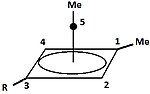
Substituted cyclopropylcarbinyl cations have also been studied by NMR:[24][25]
In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups, indicating that the molecular conformation of this cation is not perpendicular (as in A), which possesses a mirror plane, but is bisected (as in B) with the empty p-orbital parallel to the cyclopropyl ring system:
In terms of bent bond theory, this preference is explained by assuming favorable orbital overlap between the filled cyclopropane bent bonds and the empty p-orbital.[26]
Pyramidal carbocation
[edit]| Pyramidal Carbocations | ||
|---|---|---|
|
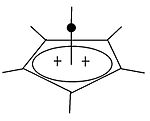
|
Besides the classical and non-classical carbocations, a third class can be distinguished: pyramidal carbocations. In these ions, a single carbon atom hovers over a four- or five-sided polygon, in effect forming a pyramid. The square pyramidal ion will carry a charge of +1, the Pentagonal pyramidal ion will carry +2.
A stable hexagonal-pyramidal configuration of tropylium trication, (C7H7)3+, has been also predicted.[27] In this case, the coordination number of carbon reaches seven. The crystal structure of [C6(CH3)6][SbF6]2·HSO3F confirms the pentagonal-pyramidal shape of the hexamethylbenzene dication.[28] |
| An example of the monovalent carbocation | An example of the divalent carbocation |
See also
[edit]References
[edit]- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 235, ISBN 978-0-471-72091-1
- ^ Robert B. Grossman (2007-07-31). The Art of Writing Reasonable Organic Reaction Mechanisms. Springer Science & Business Media. pp. 105. ISBN 978-0-387-95468-4.
- ^ Olah, George A. (1972). "Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions". Journal of the American Chemical Society. 94 (3): 808–820. doi:10.1021/ja00758a020.
- ^ Sommer, J.; Jost, R. (2000-01-01). "Carbenium and carbonium ions in liquid- and solid-superacid-catalyzed activation of small alkanes". Pure and Applied Chemistry. 72 (12): 2309–2318. doi:10.1351/pac200072122309. ISSN 1365-3075.
- ^ McMurry, John (August 1999). Organic chemistry (5th ed.). Brooks Cole. ISBN 978-0-534-37617-8.
- ^ Vollhardt, K. Peter C.; Schore, Neil Eric (2018). Organic chemistry: Structure and function (8th ed.). New York. ISBN 9781319079451. OCLC 1007924903.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Yurkanis Bruice, Paula (2004). Organic Chemistry (4th ed.). Pearson/Prentice Hall. ISBN 978-0-13-140748-0.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. ISBN 978-0-19-850346-0.
- ^ Fox, Marye Anne; Whitesell, James K. (1997). Organic Chemistry. Jones and Bartlett. ISBN 978-0-7637-0413-1.
- ^ Merling, G. (1891). "Ueber Tropin". Berichte der Deutschen Chemischen Gesellschaft. 24 (2): 3108–3126. doi:10.1002/cber.189102402151. ISSN 0365-9496.
- ^ Doering, W. von E.; Knox, L. H. (1954). "The Cycloheptatrienylium (Tropylium) Ion". Journal of the American Chemical Society. 76 (12): 3203–3206. doi:10.1021/ja01641a027.
- ^ "Discovery of an in situ carbocationic system using trityl chloride as a homogeneous organocatalyst". Tetrahedron. 69: 212–218. 2013. doi:10.1016/j.tet.2012.10.042.
- ^ Urch, C. (2001). "Triphenylmethyl Hexafluorophosphate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt363f. ISBN 0471936235.
- ^ "On the Constitution of the Salts of Imido-Ethers and other Carbimide Derivatives". American Chemical Journal. 21: 101. ISSN 0096-4085.
- ^ Meerwein, H.; Emster, K. van (1922). "About the equilibrium isomerism between bornyl chloride isobornyl chloride and camphene chlorohydrate". Berichte. 55: 2500.
- ^ Rzepa, H. S.; Allan, C. S. M. (2010). "Racemization of Isobornyl Chloride via Carbocations: A Nonclassical Look at a Classic Mechanism". Journal of Chemical Education. 87 (2): 221. Bibcode:2010JChEd..87..221R. doi:10.1021/ed800058c.
- ^ Doering, W. von E.; Saunders, M.; Boyton, H. G.; Earhart, H. W.; Wadley, E. F.; Edwards, W. R.; Laber, G. (1958). "The 1,1,2,3,4,5,6-heptamethylbenzenonium ion". Tetrahedron. 4 (1–2): 178–185. doi:10.1016/0040-4020(58)88016-3.
- ^ Story, Paul R.; Saunders, Martin (1960). "The 7-norbornadienyl carbonium ion". Journal of the American Chemical Society. 82 (23): 6199. doi:10.1021/ja01508a058.
- ^ Schleyer, Paul von R.; Watts, William E.; Fort, Raymond C.; Comisarow, Melvin B.; Olah, George A. (1964). "Stable Carbonium Ions. X.1 Direct Nuclear Magnetic Resonance Observation of the 2-Norbornyl Cation". Journal of the American Chemical Society. 86 (24): 5679–5680. doi:10.1021/ja01078a056.
- ^ Saunders, Martin; Schleyer, Paul von R.; Olah, George A. (1964). "Stable Carbonium Ions. XI.1 The Rate of Hydride Shifts in the 2-Norbornyl Cation". Journal of the American Chemical Society. 86 (24): 5680–5681. doi:10.1021/ja01078a057.
- ^ Strictly speaking, the hyperconjugative stabilization of alkyl-substituted carbocations is a type of three-center bonding. Geometrically, the C–H bonds involved in hyperconjugation are observed (or computed) to "lean" slightly toward the carbocationic center as a result (that is, the +C–C–H bond angle decreases somewhat). Nevertheless, the hydrogen atom is still primarily bonded to the carbon α to the cationic carbon. To qualify as a non-classical carbocation, the two-electron three-center bond needs to feature a group equally (or nearly equally) bonded to two electron-deficient centers. In practice, there is a continuum of possible bonding schemes, ranging from slight involvement of a neighboring group (weak hyperconjugation) to equal sharing of a group between adjacent centers (fully non-classical bonding).
- ^ Carey, Francis A. (2007). Advanced Organic Chemistry: Part A: Structure and Mechanisms. Sundberg, Richard J. (5th ed.). New York: Springer. p. 447-450. ISBN 9780387448978. OCLC 154040953.
- ^ Olah, George A.; Surya Prakash, G. K.; Rasul, Golam (July 2008). "Ab Initio/GIAO-CCSD(T) Study of Structures, Energies, and 13C NMR Chemical Shifts of C
4H+
7 and C
5H+
9 Ions: Relative Stability and Dynamic Aspects of the Cyclopropylcarbinyl vs Bicyclobutonium Ions". Journal of the American Chemical Society. 130 (28): 9168–9172. doi:10.1021/ja802445s. ISSN 0002-7863. PMID 18570420. - ^ Kabakoff, David S.; Namanworth, Eli (1970). "Nuclear magnetic double resonance studies of the dimethylcyclopropylcarbinyl cation. Measurement of the rotation barrier". Journal of the American Chemical Society. 92 (10): 3234–3235. doi:10.1021/ja00713a080.
- ^ Pittman Jr., Charles U.; Olah, George A. (1965). "Stable Carbonium Ions. XVII.1a Cyclopropyl Carbonium Ions and Protonated Cyclopropyl Ketones". Journal of the American Chemical Society. 87 (22): 5123–5132. doi:10.1021/ja00950a026.
- ^ Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A (2nd ed.).
- ^ Wang, George; Rahman, A. K. Fazlur; Wang, Bin (May 2018). "Ab initio calculations of ionic hydrocarbon compounds with heptacoordinate carbon". Journal of Molecular Modeling. 24 (5): 116. doi:10.1007/s00894-018-3640-9. ISSN 1610-2940. PMID 29696384. S2CID 13960338.
- ^ Malischewski, Moritz; Seppelt, K. (2016-11-25). "Crystal Structure Determination of the Pentagonal-Pyramidal Hexamethylbenzene Dication C
6(CH
3)2+
6". Angewandte Chemie International Edition. 56 (1): 368–370. doi:10.1002/anie.201608795. ISSN 1433-7851. PMID 27885766.
External links
[edit] Media related to Carbocations at Wikimedia Commons
Media related to Carbocations at Wikimedia Commons- Press Release The 1994 Nobel Prize in Chemistry". Nobelprize.org. 9 Jun 2010