User:Pha08rsi/Carbon Chemistry
| This is not a Wikipedia article: It is an individual user's work-in-progress page, and may be incomplete and/or unreliable. For guidance on developing this draft, see Wikipedia:So you made a userspace draft. Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |

Carbon Chemistry is the science of the non-metallic element carbon concerning properties such as its behaviour, composition and atomic structure. There are several isotopes and allotropes, of which the allotropes are significantly different from each other; these differences arise from varying molecular arrangements. It appears in various stable compounds most commonly in the hydrocarbon group, forming more compounds than any other element. In addition to its numerous properties it manifests itself in organic and inorganic forms. Carbon is the most unique element in the Universe due to the dominant role it plays in the chemistry of life.
Characteristics
[edit]Carbon has four electrons in its valence shell. Its tetravalence gives rise to numerous bonding configurations. This induces a large variation in its physical and chemical properties. Carbon occurs in both combined and elemental forms allowing the formation of many different compounds in varying size and shape. It tends to form covalent compounds. In addition to the variety of compounds that carbon forms it also occurs as isotopes.
Allotropes
[edit]
Carbon exists in various crystalline forms known as allotropes. Each allotrope has an altered molecular configuration, with diverse physical properties. Due to its unique chemical bonding properties, it has the most stable allotropes of any element. The most commonly known allotropes of carbon are: diamond, graphite and fullerenes[1] arise by natural or manufactured means.
Diamond
[edit]Diamond is one of the hardest substances known, and is used extensively in industry [2]. Diamond can be manufactured synthetically from other allotropes, but it requires extremely high temperatures and pressures to form. Its great hardness results from the fact that the entire diamond crystal is one very large molecule held together by millions of covalent bonds[3].
Each carbon atom in a diamond is covalently bonded in a tetrahedron. These tetrahedrons together form a 3-dimensional network of six-membered carbon rings. The network’s stability in its covalent bonds and hexagonal rings is the reason for the allotrope’s incredible strength. In diamond all four outer electrons of each carbon atom are 'localised' between the atoms in covalent bonding. The movement of electrons is restricted and diamond does not conduct an electric current.

Graphite
[edit]Graphite is comprised of planar layers that are weakly bounded to each other. The lattice arrangement between the carbon atoms is that of a hexagon. Due to its electron delocalisation the element has an ability to conduct electricity. It is widely distributed throughout the world. Large crystals occur in silicate rocks such as quartz and mica schists[4]. It conducts electricity in electrodes of an electrical arc lamp. Graphite holds the distinction of being the most stable form of carbon under standard conditions. Therefore, it is used in thermochemistry as the standard state for defining the heat of formation of carbon compounds.
Fullerenes
[edit]Fullerenes are a form of carbon molecule that is neither graphite nor diamond. They consist of a spherical, ellipsoid, or cylindrical arrangement of dozens of carbon atoms[5]. Fullerenes consist of 20 hexagonal and 12 pentagonal rings as the basis of an icosohedral symmetry closed cage structure[6].
Compounds
[edit]Carbon's ability to form strong bonds with itself and a variety of other elements. Organic chemistry is the study of carbon compounds with covalent bonding[7]. They bond to form open-chains or cyclic ring compounds. Saturated compounds have single carbon-carbon (C-C) atoms and unsaturated carbon compounds have multiple bonds. Isomers are a widespread occurrence in organic chemistry having the same molecular formulas but different structural formulas.

Hydrocarbons
[edit]Hydrocarbons are the simplest organic compounds . Containing only carbon and hydrogen, they can be straight-chain, branched chain, or cyclic molecules. Carbon tends to form four bonds in a tetrahedral geometry. Hydrocarbon derivatives are formed when there is a substitution of a functional group at one or more of these positions[8].
Alkanes are saturated hydrocarbons containing the maximum content of hydrogen possible[9]. There are a vast number of combinations: the simplest being methane that is comprised of only one carbon atom. Molecular arrangement differs with increase in molecular size, the smallest of these chemicals are gases, larger molecules are liquids and the largest are solids at room temperature[10]. They form a homologous series showing a gradual change in physical properties as the number of carbon atoms increases.
Unsaturated hydrocarbons are compounds that contain one double covalent bond between carbon atoms (carbon=carbon) or a triple covalent bond between carbon atoms. In these molecules, since all the bonds of carbon are not fully utilised by hydrogen atoms, more of these can be attached to them. Unsaturated hydrocarbons can be divided into 'alkenes' and 'alkynes' depending on the presence of double or triple bonds respectively.
Organic Halogen Compounds
[edit]Organic halogen compounds[11] are a large class of natural and synthetic chemicals that contain one or more halogens (fluorine, chlorine, bromine, or iodine) combined with carbon and other elements. The simplest organochlorine compound is chloromethane, also called methyl chloride. Other simple organohalogens include bromomethane, chloroform, and carbon tetrachloride. Organohalogens can be made in various ways. Direct halogenation of hydrocarbons with chlorine gives organochlorines; with bromine, organobromines. Alcohols can be converted into organohalogens by reaction with hydrogen halides.
Carbon Cycle
[edit]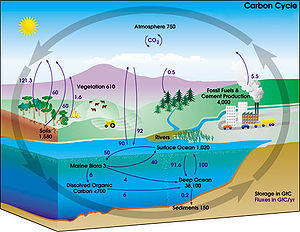
The movement of carbon, in its many forms, between the atmosphere, oceans, biosphere, and geosphere is described by the carbon cycle[12]. This cycle consists of several storage pools of carbon and the processes by which the various pools exchange carbon. Large amounts of carbon exist in the atmosphere as carbon dioxide (CO2). Carbon dioxide is cycled during photosynthesis to produce organic molecules. It is released back into the air during several processes such as respiration, decomposing of dead organic matter, the burning of fossil fuels (coal, petroleum and natural gas). Volcanoes[13] and fires also release large amounts of CO2 back into the atmosphere. The carbon cycle is imperative in ecosystems because it constantly moves carbon, from the atmosphere and oceans into organisms and vice versa. It is necessary for energy sustenance (in the form of carbohydrates).
Why is Carbon essential to life?
[edit]Considering the chemical make-up of terrestrial biology it is clear to see that carbon is a key elemental building block of life. Its abilities in forming a vast range of large complex molecules with itself and other elements (particularly hydrogen, oxygen and nitrogen) and unique facility for maintaining the right balance in molecular transformations are what underlie the complexity of life. In aqueous systems at temperatures common on Earth, carbon has come to be the basis for the structure of biomolecules essential for all basic metabolic processes. It is difficult to see viable alternatives to carbon biochemistry as so far nothing has been discovered in the chemistry of other elements. The one possible exception is silicon which is chemically similar to carbon and an important constituent of many living cells.
References
[edit]- ^ http://wiki.riteme.site/wiki/Carbon
- ^ http://www.wisegeek.com/what-are-the-allotropes-of-carbon.htm
- ^ Organic Chemistry, 5th Edition: T.W.G.Solomons, (John Wiley & Sons, Inc. Canada, 1992)
- ^ Chemistry of the Elements, 2nd Edition: N.N.Greenwood & A.Earnshaw, (Reed Educational and Professional Publishing Ltd., Oxford, 1997)
- ^ http://www.wisegeek.com/what-are-fullerenes.htm
- ^ http://www.ch.ic.ac.uk/local/projects/unwin/Fullerenes.html
- ^ Organic Chemistry, Schaum's easy outlines: H.Meislich, H.Nechamkin & J.Sharefkin, (The McGraw-Hill Companies, Inc., USA, 2000)
- ^ http://hyperphysics.phy-astr.gsu.edu/HBASE/Organic/hydrocarbon.html
- ^ Chemistry in Context: G.Hill & J.Holman, (Nelsen Thornes Ltd., United Kingdom, 2000)
- ^ http://www.purchon.com/chemistry/alkanes.htm
- ^ http://www.chemistryexplained.com/Ny-Pi/Organic-Halogen-Compounds.html
- ^ http://www.visionlearning.com/library/module_viewer.php?mid=95
- ^ http://library.thinkquest.org/11353/carbon.htm
External links
[edit]

