User:Pghaas/sandbox
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
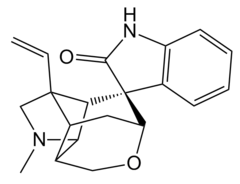
| Formula | C20H22N2O2 |
| Molar mass | 322.40 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
Gelsemine (C20H22N2O2) is an alkaloid that comes from the flowering plant, Gelsemium sempervirens, and is a highly toxic compound that acts as a paralytic and often results in death. It acts as an agonist for the glycine receptor with a significantly higher binding affinity than glycine. Recent pharmacological research has suggested that this compound can be effective in the treatment of anxiety and other conditions, and is searching for a safe way to utilize these beneficial effects.
History
[edit]While Arthur Conan Doyle is most commonly known as the mastermind behind Sherlock Holmes, he also was a doctor with a similar madness to that of Sherlock’s. Doyle’s first academic publication was concerning the effects of Gelsemium intoxication in humans. After observing the successful treatment of a gelsemium tincture for neuralgia, he became determined to “ascertain how far one might go in taking the drug, and what the primary symptoms of an overdose might be.” To do this, Doyle ingested increasing doses of his prepared tincture every day and published his results in the British Medical Journal. He determined that an adult could safely ingest up to 90 minims of the gelsemium tincture (1 minim = 1/480 fluid ounces)[1].
Synthesis
[edit]The biosynthesis of gelsemine is unknown, and synthetic synthesis proved difficult due to its complex structure containing seven stereocenters and five rings. Progress made with this molecule since its isolation in 1870. It has been synthesized seven times since 1994 by Speckamp (1994), Johnson (1994), Hart (1994), Fukuyama (1996), Overman (1999), Fukuyama (2000), and Danishefsky (2002). The only enantioselective route was Fukuyama’s 2000 synthesis[2].
Toxicology
[edit]Gelsemine has a molecular weight of 322.44 g/mol, and can be administered via ingestion or injection (subcutaneous or intraperitoneal). Its high toxicity prevents study to determine LD50 in humans, but it has been shown to have LD50s of 49mg/kg intraperitoneal in mice and 0.1mg/kg subcutaneous in rabbits.
Mechanism of Action and Symptoms
[edit]Gelsemine is an agonist for the glycine receptor (GlyR) with a much larger affinity for the receptor than glycine. When glycine receptors are activated, chloride ions enter the neuron causing an inhibitory postsynaptic potential, which causes muscle relaxation[3]. At lower doses this inhibition can cause nausea, diarrhea and muscle spasms caused by the loss of control of involuntary muscle control. Higher doses cause vision impairment or blindness, paralysis, and death[4].
Many sources have linked gelsemine with a similar alkaloid that interacts with GlyR, strychnine. It should be noted that strychnine is an antagonist of GlyR, making it a stimulatory molecule leading to dramatic muscle seizing. These two molecules work on opposition to each other.
Medicinal Applications
[edit]Gelsemium sempervirens
[edit]Although gelsemine has no historical uses as anything but a poison, the plant it comes from, Gelsemium sempervirens, has been utilized for a variety of ailments. It should be noted, though, that this plant is also toxic and there are serious safety concerns in its use[5]. Historically, Gelsemium tinctures have been a go-to treatment for a large variety of ailments. It has been utilized to treat acne and other papular eruption, anxiety, ear pain, migraine, diseases causing a large inflammatory response and in situations where someone is experiencing abnormal nervous functions (paralysis, “pins and needles” feeling, neuralgia, etc)[6][7].
Therapeutic Potential
[edit]Although gelsemine is highly toxic, it has been recognized to have therapeutic properties. Recent research has been investigating these properties in order to develop a safer gelsemine-derivative to treat a variety of disorders. The anxiolytic effects of Gelsemium sempervirens is due to gelsemine, and can be more effective in treating anxiety than Diazepam[8][9]. Gelsemine has also been found to have protective effects against oxidative stress by reducing oxidase activity and increasing anti-oxidant synthesis[10][11].
Treatment
[edit]Gelsemine is a highly toxic and typically fatal substance for which there is no antidote, but the symptoms can be managed in low dose intoxications. In the case of an oral exposure a gastric lavage is performed, which must be done within approximately one hour of ingestion. Activated charcoal is then administered to bind and eliminate free toxin in circulation. Benzodiazepine or phenobarbital will also be administered to help control seizing, and atropine can be used to treat bradycardia. Electrolyte and nutrient levels should be monitored and controlled. In the case of a skin exposure, the area should be washed wish soap and water for 15 minutes to avoid dermal damage[12].
While there is no current treatment to reverse the effects of gelsemine poisoning, preliminary research has suggested that strychnine has potential therapeutic applications due to its antagonistic effects on the glycine receptor[13].
See also
[edit]- Gelsemium sempervirens
- Strychnine
- Glycine receptor antagonists
- Alkaloids
References
[edit]- ^ Doyle, Arthur Conan (20 September 1879), "Arthur Conan Doyle takes it to the limit (1879)", British Medical Journal, BMJ Publishing Group Ltd
{{citation}}: Missing or empty|url=(help) - ^ Austin, Joel (9 January 2002), Comparative Syntheses of Gelsemine, MacMillan Group Meeting
- ^ Venard, C; et al. (2008), "Regulation of neurosteroid allopregnanolone biosynthesis in the rat spinal cord by glycine and the alkaloidal analogs strychnine and gelsemine", Neuroscience, 153 (1): 154–61, doi:10.1016/j.neuroscience.2008.02.009, PMID 18367344, S2CID 22806852
- ^ Brower, Justin (12 August 2014), Gelsemium and Sir Arthur Conan Doyle, the Self-Poisoner, Natures Poisons
- ^ Gelsemium
- ^ Winterburn, George William (1883), Gelsemium sempervirens, vol. 10, Transactions of the National Eclectic Medical Association
- ^ Gelsemium sempervirens (Gels.)
- ^ Meyer, L; et al. (2013), "Pharmacological effect of gelsemine on anxiety-like behavior in rat", Behav Brain Res, 31: 90–4, doi:10.3389/fneur.2011.00031, PMID 21647210, S2CID 130006
{{citation}}: CS1 maint: unflagged free DOI (link) - ^ Chirumbolo, S (2011), "Gelsemine and Gelsemium sempervirens L. Extracts in Animal Behavioral Test: Comments and Related Biases", Frontiers in Neurology, 31: 31, doi:10.3389/fneur.2011.00031, PMC 3098419, PMID 21647210
{{citation}}: CS1 maint: unflagged free DOI (link) - ^ Lin, L; et al. (2015), "Nephroprotective effect of gelsemine against cisplatin-induced toxicity is mediated via attenuation of oxidative stress", Cell Biochem Biophys, 71 (2): 535–41, doi:10.1007/s12013-014-0231-y, PMID 25343941, S2CID 16724810
- ^ Wu, T; et al. (2015), "Anti-hyperlipidemic and anti-oxidative effects of gelsemine in high-fat-diet-fed rabbits", Cell Biochem Biophys, 71 (1): 337–44, doi:10.1007/s12013-014-0203-2, PMID 25213292, S2CID 9777283
- ^ Gelsemine, 8 November 2002
- ^ Meyer, L; et al. (2013), "Pharmacological effect of gelsemine on anxiety-like behavior in rat", Behav Brain Res, 31: 90–4, doi:10.3389/fneur.2011.00031, PMID 21647210, S2CID 130006
{{citation}}: CS1 maint: unflagged free DOI (link)
