User:Naturwiki/sandbox
Synthesis
[edit]Several routes exist for the synthesis of Dextromethorphan. Even though many of the syntheses have been known since the middle of the 20th century, researchers are still working today to further develop the synthesis of Dextromethorphan and, for example, to make it more environmentally friendly.
This includes the synthesis by means of ionic liquids.[1]
Racemate separation
[edit]Since only one of the stereoisomers has the desired effect, the separation of a racemic mixture of hydroxy N- methyl morphinan using tartaric acid and subsequent methylation of the hydroxyl group is a suitable method. By using (D)-tartrate, the (+)-isomer remains as the product.
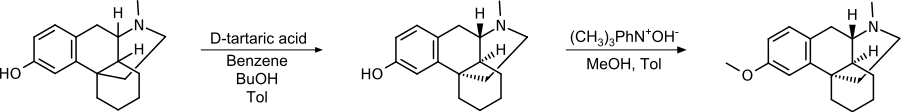
This synthetic pathway was patented by Roche in 1950.
Traditional synthesis
[edit]The traditional synthetic route uses Raney nickel and has been further improved over time, for example by the use of ibuprofen and ACl3.

Overall, it is a cost-effective method with moderate reaction conditions that is easy to handle and suitable for industrial production.[1]
Grewe's cyclization
[edit]
Grewe's cyclization is easier to handle in terms of the chemicals used, produces higher yields and higher purity of the product.[2]
Improved Grewe's cyclization
[edit]Formylation prior to cyclization avoids ether cleavage as a side reaction and yields higher than without N-substitution or N-methylation. In this example, the purification was done by formation of a brucine salt.[1]

This process has also been patented by Roche.
- ^ a b c Sethi, Madhuresh Kumar (2018-06-11). "Preparation of Morphine Derivatives Using Ionic Liquids". Archives of Organic and Inorganic Chemical Sciences. 3 (2). doi:10.32474/AOICS.2018.03.000156.
- ^ MEUZELAAR, G. J.; NEELEMAN, E.; MAAT, L.; SHELDON, R. A. (2010-06-17). "ChemInform Abstract: A Novel Synthesis of Substituted 1-Benzyloctahydroisoquinolines by Acid-Catalyzed Cyclization of N-[2-(Cyclohex-1-enyl)ethyl]-N-styrylformamides". ChemInform. 30 (5): no–no. doi:10.1002/chin.199905129. ISSN 0931-7597.

