User:Mr. Ibrahem/Ledipasvir/sofosbuvir
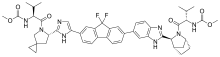 Ledipasvir | |
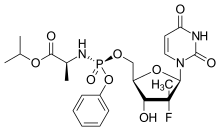 Sofosbuvir structure | |
| Combination of | |
|---|---|
| Ledipasvir | NS5A inhibitor |
| Sofosbuvir | NS5B (RNA polymerase) inhibitor |
| Clinical data | |
| Trade names | Harvoni, Hepcinat-LP, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C71H83F3N11O15P |
| Molar mass | 1418.476 g/mol |
| Melting point | 170–225 °C (338–437 °F) |
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C.[2] It is a fixed-dose combination of ledipasvir and sofosbuvir.[2] Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1.[4] Some evidence also supports use in HCV genotype 3 and 4.[4] It is taken daily by mouth for 8–24 weeks.[2]
It is generally well tolerated.[5] Common side effects include muscle pains, headache, nausea, rash, and cough.[2] It is unclear if use in pregnancy is safe for the baby.[2] Ledipasvir works by decreasing the activity of NS5A and sofosbuvir works by decreasing the activity of NS5B polymerase.[2]
Ledipasvir/sofosbuvir was approved for medical use in the United States in 2014.[2] It is on the World Health Organization's List of Essential Medicines.[6] The wholesale cost in the United States is about US$91,589.40 for 12 weeks as of 2016.[7] In Bangladesh this amount costs US$1,092.00.[8] Some people travel to India to get access to lower cost medication.[9]
References
[edit]- ^ a b "Ledipasvir / sofosbuvir (Harvoni) Use During Pregnancy". Drugs.com. 28 October 2019. Archived from the original on 17 March 2020. Retrieved 17 March 2020.
- ^ a b c d e f g h i "Ledipasvir and Sofosbuvir". The American Society of Health-System Pharmacists. Archived from the original on 25 December 2016. Retrieved 8 December 2016.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 3 August 2020. Retrieved 16 September 2020.
- ^ a b Keating GM (2015). "Ledipasvir/Sofosbuvir: a review of its use in chronic hepatitis C". Drugs. 75 (6): 675–85. doi:10.1007/s40265-015-0381-2. PMID 25837989.
- ^ World Health Organization (2015). The selection and use of essential medicines. Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. pp. 69–70. hdl:10665/189763. ISBN 9789241209946. ISSN 0512-3054. WHO technical report series;994.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "NADAC as of 2016-12-21 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 24 December 2016. Retrieved 25 December 2016.
- ^ Azam, Monirul (2016-05-30). "1". Intellectual Property and Public Health in the Developing World. Open Book Publishers. ISBN 9781783742318. Archived from the original on 2016-12-26.
- ^ "Hep C drug tourism has begun as patients seek Harvoni, Sovaldi overseas". FiercePharma. 2015-06-02. Archived from the original on 2015-11-04. Retrieved 2015-10-25.
