User:Michael7604/Bis(4-bromobenzyl) ketone
Appearance
The topic of this article may not meet Wikipedia's general notability guideline. (January 2023) |
| |

| |

| |
| Names | |
|---|---|
| Systematic IUPAC name
1,3-bis(4-bromophenyl)propan-2-one | |
| Other names
1,3-bis(4-bromophenyl)-2-propanone
1,3-bis(4-bromophenyl)acetone 4,4'-dibromodibenzyl ketone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H12Br2O | |
| Molar mass | 368.06 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tracking categories (test):
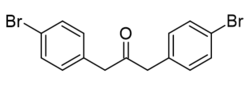
Bis(4-bromobenzyl) ketone is a variation of dibenzyl ketone containing 2 bromine atoms at the para positions of the phenyl rings.
Preparation
[edit]Bis(4-bromobenzyl) ketone may be prepared in the laboratory by ketonic decarboxylation of 4-bromophenylacetic acid using DCC and DMAP.[1]
Reactions
[edit]Bis(4-bromobenzyl) ketone reacts with benzil in the presence of base in a double aldol condensation to form 2,5-bis(4-bromophenyl)-3,4-diphenylcyclopentadienone.[2] As this tetraphenylcyclopentadienone derivative may be condensed with diphenylacetylenes, this makes it a useful precursor to symmetrical hexaphenylbenzene and coronene derivatives.[3][4]
References
[edit]- ^ Bhandari, Sumita; Ray, Suprabhat (17 Jun 1997). "A Novel Synthesis of Bisbenzyl Ketones by DCC Induced Condensation of Phenylacetic Acid". Synthetic Communications. 28 (5): 765–771. doi:10.1080/00032719808006472.
- ^ Coan, Stephen B.; Trucker, Donald E.; Beckerr, Ernest I. (1955). "The Absorption Spectra of Tetracyclones. IV". Journal of the American Chemical Society. 77 (1): 60–66. doi:10.1021/ja01606a018.
- ^ Soichi, Watanabe; Junji, Kido (2007). "Hexaphenylbenzene Derivatives for Blue Organic Light-emitting Devices". Chemistry Letters. 36 (5): 590–591. doi:10.1246/cl.2007.590.
- ^ Ito, Shunji; Wehmeier, Mike; Brand, J. Diedrich; Kübel, Christian; Epsch, Rebekka; Rabe, Jürgen P.; Müllen, Klaus (2000). "Synthesis and Self-Assembly of Functionalized Hexa-peri-hexabenzocoronenes". Chemistry: A European Journal. 6 (23): 4327–4342. doi:10.1002/1521-3765(20001201)6:23<4327::AID-CHEM4327>3.0.CO;2-7. PMID 11140962.
