User:Mdlevin/sandbox

Organogold compounds are chemical compounds that contain one or more chemical bond between carbon and gold. Organogold chemistry is the corresponding science exploring properties, structure, and reactivity of these compounds.
In line with inorganic gold chemistry, the organometallic chemistry of gold is typified by compounds in the +1 and +3 oxidation state, although examples involving the +2 oxidation state have also been explored.[1][2][3]
Gold chemistry is characterized by the high electronegativity and oxidation potential of gold. This results in a tendency for organometallic compounds of gold(III) to undergo both nonspecific reduction as well as reductive elimination, making their isolation more difficult than the corresponding gold(I) compounds.[4] A second hallmark of gold chemistry is the aurophilic effect, which results in the aggregation of gold atoms in polynuclear compounds.
The air and water tolerance of organogold compounds has led to intense academic research regarding their use as catalysts for organic synthesis. The luminescence properties of many organogold complexes have been explored in academic settings as well. However, in both arenas, the high cost of gold has limited broad adoption. Several reviews detailing these applications have been published.
Synthesis and Structure
[edit]Gold(I)
[edit]Gold(I) complexes are typically 2-coordinate, linear, diamagnetic, 14 electron species. They generally exist as neutral adducts LAuX, with a dative ligand L (for instance a triphenylphosphine or an isocyanide) and an anionic ligand X. Gold(I) can also exist as the aurate M+AuX2- and as cationic L2Au+X- complexes, both of which are also typically linear.
In many instances, gold(I) will adopt higher coordination numbers (typically 3 or 4), depending on the nature of the coordinating ligand. Often, these complexes are only tri- or tetra-coordinate in the solid state, with ligand dissociation occuring upon dissolution.
π complexes
[edit]Organic ligands containing unsaturation form complexes with gold as L-type ligands. Both cationic and neutral complexes of this type have been isolated. Several examples are shown below. Several studies have supported the conclusion that this interaction is donated by σ donation from the ligand π system and do not experience significant π backbonding. (See Dewar–Chatt–Duncanson model.) These complexes can generally be synthesized directly from the π ligand and the corresponding cationic LAu+ species or chloro(dimethyl sulfide)gold(I).

σ-bonded complexes
[edit]Organic ligands also form complexes with gold as X-type ligands. Alkyl (sp3), Aryl (sp2), and Alkynyl (sp) complexes have all been isolated. Typical syntheses employ Grignard or organolithium reagents and the corresponding gold halide complex, though transmetallation from boronic acids is also commonly employed. Other methods include decarboxylation of gold carboxylate complexes and attack on coordinated π-ligands. (See Attack on Coordinated Ligands below)
These complexes typically require a supporting phosphine, carbene, or other L-type ligand to prevent decomposition, with the exception of -ate complexes and those which form oligomeric bridging complexes such as (MesAu)5. (See Reductive Homocoupling below)

Miscellaneous complexes
[edit]In addition to the two major types of organogold complexes outlined above, there exist a number of complexes where the nature of the interaction between the metal center and the organic ligand is less well-defined. Emblematic of such compounds are carbene complexes of gold, which have been prepared with a variety of appended functional groups which modulate their electronic properties. In addition to N-heterocyclic carbene complexes of gold which are routinely prepared, carbenoid-like, carbenium-like, and alkylidene-like complexes have all been characterized. The nuances of this continuum have been the subject of several reviews. These complexes can be prepared from diazo compounds, via transmetallation from chromium Fischer carbene complexes, or by hydride abstraction from alkyl complexes.

In addition to these complexes, complexes of carbon monoxide and isonitriles have been prepared. These behave as L-type ligands on gold and are generally weakly coordinating due to weak backbonding. Gold cyanide compounds (MAu(CN)2) are of some importance to gold cyanidation, a process for the extraction of gold from low-grade ore. The carbon to metal bond in metal cyanides is usually ionic but evidence exists that the C-Au bonding in the gold cyanide ion is covalent.[5]
More unusual compounds include the carbide complex [Au6C(PPh3)6]2+ shown above, in which six aurophilically bonded gold atoms surround a central carbide ligand. LX-type bidentate bisylide ligands [(CH2)2PR2]- can also form unusual organogold complexes in which two gold atoms are ligated in an A-frame complex.
Gold(III)
[edit]Gold(III) complexes are typically 4 coordinate, square planar, diamagnetic, 16 electron species. When the formal coordination number is less than 4, bridged dimers and oligomers form.
Other oxidation states
[edit]Reactivity
[edit]Stoichiometric
[edit]- Attack on Coordinated Ligand
- Reductive Homocoupling
- Protodeauration
- Oxidative Addition
- Reductive Elimination
- Migratory Insertion
Unlike other transition metal alkene complexes, gold π-complexes do not undergo intramolecular migratory insertion reactions, rather undergoing outer-sphere nucleophilic attack.
Catalytic
[edit]Gold-catalyzed reactions fall into two major categories: heterogeneous catalysis (including gold nanoparticles and thiol-monolayer gold surfaces), and homogeneous catalysis that occurs with gold(I) or gold(III) compounds and is used for organic synthesis.[6] Common commercially available gold catalysts are gold(I) chloride, gold(III) chloride, chloroauric acid and a range of gold phosphines such as chloro(triphenylphosphine)gold(I) . To increase the electrophilicity at the gold center, a more dissociated complex can be created through halide abstraction with an additive like silver triflate (AgOTf).
Gold is certainly not the only metal showing this type of bonding and reactivity, several metal ions isolobal with the simple hydrogen proton (cationic: emptied s-orbital) do as well: mercury(II) and platinum(II). In 2007 Fürstner & Davies [7] proposed the term pi-acid as a cognomen for this type of ion (See also: cation-pi interaction).
The resulting metal-multiple bond complex is electrophilic in nature and activated for nucleophilic attack. In oxymercuration the resultant organomercurial species is generated stoichiometrically, and requires an additional step to liberate the product. Facile protonolysis of the Au-C bond enables true catalysis. Other advantages of gold(I) catalysis include: air stability (high oxidation potential of Au(I) to Au(III) is a barrier to oxidation), lowered oxophilicity and thus tolerance towards water and alcohols, C-Au bonds that prefer protodeauration over beta-hydride elimination, and relative non-toxicity.[8]
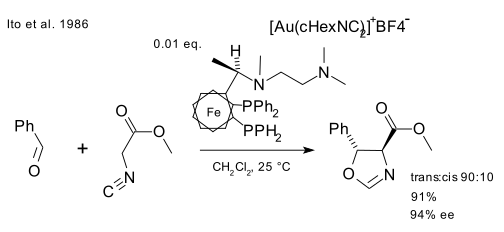
An early example of catalysis by gold was described by Ito in 1986 [9] who reacted benzaldehyde and methyl isocyanoacetate with a chiral ferrocenylphosphine ligand and cationic gold(I) compound (Au(cHexCN)2)BF4 to form a chiral oxazoline. This reaction was a first in more than one way: asymmetric Aldol reactions already existed but this was also the first catalytic one.
In a simple mechanistic picture, gold(I) simultaneously coordinates to two phosphine ligands and the carbon isocyanate group [10] which is then attacked by the carbonyl group. Further studies on the bonding mode of Au(I) indicate that this simple picture may have to be revised.
In 1976, Thomas and coworkers reported conversion of phenylacetylene to acetophenone using tetrachloroauric acid in a 37% yield.[11] In this reaction gold(III) was used as a homogeneous catalyst replacing mercury in oxymercuration. Interestingly, this same study lists a published yield >150%, indicating catalysis that perhaps was not acknowledged by the chemists.
In 1991 Utimoto reacted gold(III)(NaAuCl4) with alkynes and water.[12] Teles identified a major drawback of this method as Au(III) was rapidly reduced to catalytically dead metallic gold and in 1998 returned to the theme of ligand supported Au(I) for the same transformation:[13]
This particular reaction would trigger a lot of academic interest in gold catalysis in the years to come [14]
Another example of an Au(III) catalysed reaction (Hashmi et al. 2000) is in a forced alkyne / furan Diels-Alder reaction - these do not normally occur - ultimately forming a phenol:[15]
Further mechanistic studies conclude that this is not a concerted transformation, but rather an initial alkyne hydroarylation followed by a series of non-obvious intramolecular rearrangements, concluded with a 6-pi electrocyclization and rearomatization.
Heterogeneous gold catalysis is an older science. Gold is an attractive metal to use because of its stability against oxidation and its variety in morphology for instance gold cluster materials. Gold has been shown to be effective in low-temperature CO oxidation and acetylene hydrochlorination to vinyl chlorides. The exact nature of the catalytic site in this type of process is debated.[16]
Gold catalyzes many organic transformations, usually carbon-carbon bond formation from Au(I), and C-X (X = O, N) bond formation from the Au(III) state, due to that ion's harder Lewis acidity. [17]
- heteroatom nucleophilic addition to unsaturated C-C bonds, especially to form small heterocycles (furans, pyrroles, thiophenes)
- Hydroarylation: basically a Friedel-Crafts reaction using metal-alkyne complexes. Example, the reaction of mesitylene with phenylacetylene:[18]
- Enyne cyclization, in particular cycloisomerization, one early example being an 5-exo-dig 1,6 enyne cycloisomerization:[19]
- The soft gold(I) ion coordinates exclusively to the more pi-basic alkyne, leaving the alkene free to attack as the nucleophile.
- cycloaddition reactions with early example the cycloaddition of an nitrile oxide with an alkyne.[20]
Other reactions are the use of gold in C-H bond activation [21] and Itoh Aldol reactions (1990's). Gold also catalyses coupling reactions.[22]
References
[edit]- ^ C. Elschenbroich, A. Salzer Organometallics : A Concise Introduction (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ Organogold Chemistry: II Reactions R.V. Parish Gold Bulletin 1997 Link
- ^ Organogold Chemistry: III Applications R.V. Parish Gold Bulletin 1997 Link
- ^ Wiberg, Egon; Wiberg, Nils and Holleman, Arnold Frederick (2001). Inorganic Chemistry (101 ed.). Academic Press. p. 1286. ISBN 0-12-352651-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Evidence of Significant Covalent Bonding in Au(CN)2− Xue-Bin Wang, Yi-Lei Wang, Jie Yang, Xiao-Peng Xing, Jun Li and Lai-Sheng Wang J. Am. Chem. Soc., 2009, 131 (45), pp 16368–16370 doi:10.1021/ja908106e
- ^ Gold catalysis for organic synthesis F. Dean Toste (Editor) Thematic Series in the Open Access Beilstein Journal of Organic Chemistry
- ^ Review Catalytic Carbophilic Activation: Catalysis by Platinum and Gold Acids Alois Fürstner , Paul W. Davies Angewandte Chemie International Edition 2007 Volume 46 Issue 19, Pages 3410 - 3449 doi:10.1002/anie.200604335
- ^ Recent advances in syntheses of heterocycles and carbocycles via homogeneous gold catalysis. Part 1: Heteroatom addition and hydroarylation reactions of alkynes, allenes, and alkenes Hong C. Shen Tetrahedron Volume 64, Issue 18, 28 April 2008, Pages 3885-3903 doi:10.1016/j.tet.2008.01.081
- ^ Catalytic asymmetric aldol reaction: reaction of aldehydes with isocyanoacetate catalyzed by a chiral ferrocenylphosphine-gold(I) complex Yoshihiko. Ito, Masaya. Sawamura, Tamio. Hayashi J. Am. Chem. Soc., 1986, 108 (20), pp 6405–6406 doi:10.1021/ja00280a056
- ^ Chiral cooperativity: the nature of the diastereoselective and enantioselective step in the gold(I)-catalyzed aldol reaction utilizing chiral ferrocenylamine ligands Antonio Togni, Stephen D. Pastor J. Org. Chem., 1990, 55 (5), pp 1649–1664 doi:10.1021/jo00292a046
- ^ The reactions of alkynes, cyclopropanes, and benzene derivatives with gold(III) Richard O. C. Norman, William J. E. Parr and C. Barry Thomas J. Chem. Soc., Perkin Trans. 1, 1976, 1983 - 1987, doi:10.1039/P19760001983
- ^ Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst' Yakitoshi Fukuda, Kiitiro Utimoto J. Org. Chem., 1991, 56 (11), pp 3729–3731 doi:10.1021/jo00011a058
- ^ Communication Cationic Gold(I) Complexes: Highly Efficient Catalysts for the Addition of Alcohols to Alkynes J. Henrique Teles , Stefan Brode, Mathieu Chabanas Angewandte Chemie International Edition Volume 37 Issue 10, Pages 1415 - 1418 1998 Link
- ^ Nugent, W. A. (2012), “Black Swan Events” in Organic Synthesis . Angew. Chem. Int. Ed., 51: 8936–8949. doi:10.1002/anie.201202348
- ^ Highly Selective Gold-Catalyzed Arene Synthesis A. Stephen K. Hashmi, Tanja M. Frost, and J. W. Bats J. Am. Chem. Soc., 2000, 122 (46), pp 11553–11554 doi:10.1021/ja005570d
- ^ Gold—an introductory perspective Graham J. Hutchings, Mathias Brust and Hubert Schmidbaur Chem. Soc. Rev., 2008, 37, 1759 - 1765, doi:10.1039/b810747p
- ^ Recent advances in syntheses of carbocycles and heterocycles via homogeneous gold catalysis. Part 2: Cyclizations and cycloadditions Hong C. Shen Tetrahedron Volume 64, Issue 34, 18 August 2008, Pages 7847-7870 doi:10.1016/j.tet.2008.05.082
- ^ Gold-Catalyzed Hydroarylation of Alkynes Manfred T. Reetz, Knut Sommer European Journal of Organic Chemistry Volume 2003 Issue 18, Pages 3485 - 3496 doi:10.1002/ejoc.200300260
- ^ Communication Cationic Gold(I) Complexes: Highly Alkynophilic Catalysts for the exo- and endo-Cyclization of Enynes Cristina Nieto-Oberhuber, M. Paz Muñoz, Elena Buñuel, Cristina Nevado, Diego J. Cárdenas, Antonio M. Echavarren Angewandte Chemie International Edition Volume 43 Issue 18, Pages 2402 - 2406 2004 doi:10.1002/anie.200353207
- ^ Gold(III)-catalyzed one-pot synthesis of isoxazoles from terminal alkynes and nitric acid Francesco Gasparrini, Mario Giovannoli, Domenico Misiti, Giovanni Natile, Gianni Palmieri, Luciana Maresca J. Am. Chem. Soc., 1993, 115 (10), pp 4401–4402 doi:10.1021/ja00063a084
- ^ The golden gate to catalysis Anja Hoffmann-Röder and Norbert Krause Org. Biomol. Chem., 2005, 3, 387 - 391, doi:10.1039/b416516k
- ^ Wegner, H. A. and Auzias, M. (2011), Gold for C[BOND]C Coupling Reactions: A Swiss-Army-Knife Catalyst?. Angewandte Chemie International Edition, 50: 8236–8247. doi:10.1002/anie.201101603
See also
[edit]- Other chemistries of carbon with other elements in the periodic table.