User:MTLE4470 grp8 fbpw/sandbox
 | This is a user sandbox of MTLE4470 grp8 fbpw. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Chitin
[edit]See: Chitin
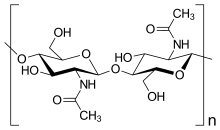
Chitin is the second most abundant natural polymer in the world, with collagen being the first. It is a “linear polysaccharide of β-(1-4)-2-acetamido-2-deoxy-D-glucose”. Chitin is highly crystalline and is usually composed of chains organized in a β sheet. Due to its high crystallinity and chemical structure, it is insoluble in many solvents. It also has a low toxicity in the body and is inert in the intestines. Chitin also has antibacterial properties.[1]
Chitin forms crystals that make fibrils that become surrounded by proteins. These fibrils can bundle to make larger fibers that contribute to the hierarchical structure of many biological materials.[2] These fibrils can form randomly oriented networks that provide the mechanical strength of the organic layer in different biological materials.[3]
Chitin provides protection and structural support to many living organisms. It makes up the cell walls of fungi and yeast, the shells of mollusks, the exoskeletons of insects and arthropods. In shells and exoskeletons, the chitin fibers contribute to their hierarchical structure.[4]
In nature, pure chitin (100% acetylation) does not exist. It instead exists as a copolymer with chitin's deacetylated derivative, chitosan. When the acetylized composition of the copolymer is over 50% acetylated it is chitin.[2] This copolymer of chitin and chitosan is a random or block copolymer.[4]
Chitosan
[edit]See: Chitosan

Chitosan is a deacetylated derivative of chitin. When the acetylized composition of the copolymer is below 50% it is chitosan.[2] Chitosan is a semicrystalline “polymer of β-(1-4)-2-amino-2-deoxy-D-glucose”.[4] One difference between chitin and chitosan is that chitosan is soluble in acidic aqueous solutions. Chitosan is easier to process that chitin, but it is less stable because it is more hydrophilic and has pH sensitivity. Due to its ease of processing, chitosan is used in biomedical applications.[1]
Properties
[edit]Compared to synthetic fibers, natural fibers tend have decreased stiffness and strength.[4]
| Material | Fiber | Elastic Modulus (GPa) | Strength (MPa) |
|---|---|---|---|
| Tendon | Collagen | 1.50 | 150 |
| Bone | Collagen | 20.0 | 160 |
| Mud Crab Exoskeleton (wet) | Chitin | 0.48 | 30 |
| Prawn Exoskeleton (wet) | Chitin | 0.55 | 28 |
| Bovine Hoof | Keratin | 0.40 | 16 |
| Wool | Keratin | 0.50 | 200 |
Properties also decrease with the age of the fiber. Younger fibers tend to be stronger and more elastic than older ones.[4] Many natural fibers exhibit strain rate sensitivity due to their viscoelastic nature.[5] Bone contains collagen and exhibits strain rate sensitivity in that the stiffness increases with strain rate, also know as strain hardening. Spider silk has hard and elastic regions that together contribute to its strain rate sensitivity, these cause the silk to exhibit strain hardening as well.[2] Properties of natural fibers are also dependent on the moisture content in the fiber.[4]
References
[edit]- ^ a b Rinaudo, Marguerite (2006-07-01). "Chitin and chitosan: Properties and applications". Progress in Polymer Science. 31 (7): 603–632. doi:10.1016/j.progpolymsci.2006.06.001.
- ^ a b c d Meyers, Marc André; Chen, Po-Yu; Lin, Albert Yu-Min; Seki, Yasuaki (2008-01-01). "Biological materials: Structure and mechanical properties". Progress in Materials Science. 53 (1): 1–206. doi:10.1016/j.pmatsci.2007.05.002.
- ^ Meyers, Marc A.; Chen, Po-Yu; Lopez, Maria I.; Seki, Yasuaki; Lin, Albert Y. M. (2011-07-01). "Biological materials: A materials science approach". Journal of the Mechanical Behavior of Biomedical Materials. Special Issue on Natural Materials / Papers from the Third International Conference on the Mechanics of Biomaterials and Tissues. 4 (5): 626–657. doi:10.1016/j.jmbbm.2010.08.005.
- ^ a b c d e f g Meyers, M.A.; Chen, P.Y. (2014). Biological Materials Science. United Kingdom: Cambridge University Press.
- ^ Fratzl, Peter; Weinkamer, Richard (2007-11-01). "Nature's hierarchical materials". Progress in Materials Science. 52 (8): 1263–1334. doi:10.1016/j.pmatsci.2007.06.001.
