User:Kcsunshine999/sandbox
 | This is a user sandbox of Kcsunshine999. A user sandbox is a subpage of the user's user page. It serves as a testing spot and page development space for the user and is not an encyclopedia article. |
user:KCsunshine999 sandbox
| Names | |
|---|---|
| IUPAC name
nickel cyclam
| |
| Other names
tetraazacyclotetradecane
| |
| Identifiers | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
μ
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.[1]
As a mix of biochemistry and inorganic chemistry, bioinorganic chemistry is important in elucidating the implications of electron-transfer proteins, substrate bindings and activation, atom and group transfer chemistry as well as metal properties in biological chemistry.


https://commons.wikimedia.org/wiki/File:TEST_figure_for_practice_uploading_(M_transport)_F15.pdf
| KC practice infobox | |
|---|---|
 | |
| Identifiers | |
| Symbol | KC infobox |
| Element | Polyhedron | Number of Faces | Number of Triangles | |

| |

| |
| Names | |
|---|---|
| IUPAC name
1,4,8,11-Tetraazacyclotetradecane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C10H24N4 | |
| Molar mass | 200.330 g·mol−1 |
| Melting point | 185 to 188 °C (365 to 370 °F; 458 to 461 K) |
| 5 g/100 mL (20 °C) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
sdfsf
 α-D-glucopyranose (chair form)
| |
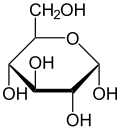 Haworth projection of α-D-glucopyranose
| |
 Fischer projection of D-glucose
| |
| Names | |
|---|---|
| Pronunciation | /ˈɡluːkoʊz/, /ˈɡluːkoʊs/ |
| Preferred IUPAC name
D-Glucose | |
| Systematic IUPAC name
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal | |
| Other names
Blood sugar
Dextrose Corn sugar D-Glucose Grape sugar | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| Abbreviations | Glc |
| 1281604 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 83256 | |
| KEGG | |
| MeSH | Glucose |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
| Appearance | White powder |
| Density | 1.54 g/cm3 |
| Melting point | α-D-glucose: 146 °C (295 °F; 419 K) β-D-glucose: 150°C (302°F; 423 K) |
| 909 g/L (25 °C (77 °F)) | |
| −101.5×10−6 cm3/mol | |
| 8.6827 | |
| Thermochemistry | |
Heat capacity (C)
|
218.6 J K−1 mol−1[2] |
Std molar
entropy (S⦵298) |
209.2 J K−1 mol−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−1271 kJ/mol[3] |
| 2,805 kJ/mol (670 kcal/mol) | |
| Pharmacology | |
| B05CX01 (WHO) V04CA02 (WHO), V06DC01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | ICSC 08655 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chemical Formula and Symbols (Visual Editor >> Insert >> more >> Chemical Formula
[edit]

: insert>>template>>underset then box 2 is for top text and box 1 is for bottom text, thereafter click on the text to "edit"
Example of Undersetting from the "Photosynthesis" article
[edit]The general equation for photosynthesis as first proposed by Cornelis van Niel is therefore:[4]
- + + → + +
Since water is used as the electron donor in oxygenic photosynthesis, the equation for this process is:
- + + → + +
This equation emphasizes that water is both a reactant in the light-dependent reaction and a product of the light-independent reaction, but canceling n water molecules from each side gives the net equation:
- + + → +
Other processes substitute other compounds (such as arsenite) for water in the electron-supply role; for example some microbes use sunlight to oxidize arsenite to arsenate:[5] The equation for this reaction is:
- + + → + (used to build other compounds in subsequent reactions)[6]
Photosynthesis occurs in two stages. In the first stage, light-dependent reactions or light reactions capture the energy of light and use it to make the energy-storage molecules ATP and NADPH. During the second stage, the light-independent reactions use these products to capture and reduce carbon dioxide.
UOIT Wikipedia Training (Jonathan Obar) F15
[edit]if you are unfamiliar with the syntax, you can use the toolbar at the top B = bold I = italics
link symbol = make a link (internal or external)
I like the Banana article. you can compel others to start a new article by creating a red link to the missing article The yellow curvy fruit article
to make an external link, just use square brackets with the URL first then the title text RCSB Protein Data Bank
to make a heading, click "advanced" then "Heading" drop down list
Level 2 Heading
[edit]Level 3 Heading
[edit]How to cite references
[edit]Visual Editor (current way to do it):
[edit]make a reflist with the heading "references" below the list of articles (use the visual editor "cite" and "insert/references list" buttons, but note that the wikimarkup syntax is in the wikimarkup quick reference "cheatsheet".
"Edit Source" Syntax (old way)
[edit]type "References" with == on either side to make the heading for a reference list at the bottom of the page
type "reflist" with double brackets {{ }} on either side of it which collects all the references into a list
References
[edit]- ^ Stephen J. Lippard, Jeremy M. Berg, Principles of Bioinorganic Chemistry, University Science Books, 1994, ISBN 0-935702-72-5
- ^ a b Boerio-Goates, Juliana (1991), "Heat-capacity measurements and thermodynamic functions of crystalline α-D-glucose at temperatures from 10K to 340K", J. Chem. Thermodynam., 23 (5): 403–09, doi:10.1016/S0021-9614(05)80128-4
- ^ Ponomarev, V. V.; Migarskaya, L. B. (1960), "Heats of combustion of some amino-acids", Russ. J. Phys. Chem. (Engl. Transl.), 34: 1182–83
- ^ Whitmarsh J, Govindjee (1999). "Chapter 2: The Basic Photosynthetic Process". In Singhal GS, Renger G, Sopory SK, Irrgang KD, Govindjee (eds.). Concepts in Photobiology: Photosynthesis and Photomorphogenesis. Boston: Kluwer Academic Publishers. p. 13. ISBN 978-0-7923-5519-9.
- ^ Anaerobic Photosynthesis, Chemical & Engineering News, 86, 33, August 18, 2008, p. 36
- ^ Kulp TR, Hoeft SE, Asao M, Madigan MT, Hollibaugh JT, Fisher JC, Stolz JF, Culbertson CW, Miller LG, Oremland RS (Aug 2008). "Arsenic(III) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California". Science. 321 (5891): 967–970. Bibcode:2008Sci...321..967K. doi:10.1126/science.1160799. PMID 18703741. S2CID 39479754.
then select "cite" at the top toolbar, then "templates" then "cite web" or "cite journal"
moving from sandbox
using talk pages: politely explain the issue you have with existing content and cite the Wikipedia policy involved. go to talk page and select "new section" to add your contribution Greetings, I wold like to add a section to this article about iron clusters. I feel that the X section as it currently exists violates TTD: add reference to Wikipedia neutral point of view article WP:NPV, or use the "link" button in toolbar Neutral point of view then sign your username using the sign button at the top toolbar of the talk page.
Hi Kcsunshine999! Thanks for contributing to Wikipedia. Be our guest at the Teahouse! The Teahouse is a friendly space where new editors can ask questions about contributing to Wikipedia and get help from peers and experienced editors. I hope to see you there! Jtmorgan (I'm a Teahouse host) This message was delivered automatically by your robot friend, HostBot (talk) 17:20, 16 October 2015 (UTC) |
Articles For My Students to Edit
[edit]- Article name here – New article
- Article name here – New article
- Article name here – New article
- Article name here – New article
- Article name here – New article
- Entatic state – Existing article (Include section that needs editing)
- Article name here – Existing article (Include section that needs editing)
- Article name here – Stub
- Article name here – Stub
- Article name here – Wikiproject article
Assignment Description
[edit]This assignment asks you to find TEN Wikipedia articles for your students to create/edit. You will post your list of TEN in your Wikipedia sandbox.
Instructions
[edit]1) The ten articles should include the following:
[edit]- 5 new articles (articles that don’t exist in the encyclopedia)
- 2 existing articles that need to be edited
- 2 stubs
- 1 wikiproject article
2) Once you find your articles, please add internal links to your sandbox linking to the five articles. The code should look like this:
[edit]* [[Article name here]] – New article
* [[Article name here]] – New article
* [[Article name here]] – New article
* [[Article name here]] – New article
* [[Article name here]] – New article
* [[Entatic state]] – Existing article (Include section that needs editing)
* [[Article name here]] – Existing article (Include section that needs editing)
* [[Article name here]] – Stub
* [[Article name here]] – Stub
* [[Article name here]] – Wikiproject article
NOTE: “Article name” can be found at the top of the article. For example “Banana” is the article name for the banana article.
3) Searching for articles:
[edit]New Articles
[edit]- This may be tough! Start by opening up a syllabus or some slides on your computer and find concepts that you teach in class. To complete this component of the assignment quickly, choose concepts that aren’t very popular, or are rather complex.
- Search for new articles by entering article names into the Wikipedia search bar.
- Once you search, you want to see the text “You may create the page (your article name here).” The article name should be a red internal link.
Existing Article
[edit]- Using the search bar again, look for articles that relate to your classes.
- Find existing articles that need work. Add them to your sandbox.
- Note the section of the article that needs editing.
Stub
[edit]- Stub articles have been started but need a lot of work.
- Search the stub lists here: https://wiki.riteme.site/?title=Wikipedia:WikiProject_Stub_sorting/Stub_types
- Find a stub that needs work and add it to your sandbox.
Wikiproject Article
[edit]- Use Google to find a wikiproject related to your discipline.
- To find a wikiproject, try searching for “wikiproject (your discipline)”.
- Once you find a wikiproject, review the page.
- Find an article for your students to work on.
- It may be easiest to find a link to relevant stubs on the wikiproject page, though some wikiprojects include articles that need editing on their home page.
Please let me know if you have any questions.


