User:Jukaredaa/sandbox
 | This is a user sandbox of Jukaredaa. A user sandbox is a subpage of the user's user page. It serves as a testing spot and page development space for the user and is not an encyclopedia article. |
Group project: Endrin
Group members include DeusetScientia and Tracklete14
This sandbox will be used as a draft article for the topic
Bibliography
[edit]- "Endrin" on TOXNET of U.S. National Library of Medicine TOXNET
- "Endrin" on GESTIS Substance Database of IFA GESTIS
- "Toxicological Profile For Endrin" by US Department of Health and Human Services CDC
- http://ces.iisc.ernet.in/energy/HC270799/HDL/ENV/enven/vol338.htm
- http://www.speclab.com/compound/c72208.htm
- http://webbook.nist.gov/cgi/cbook.cgi?ID=C72208&Units=SI&Mask=6F
- http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi
- http://www.chemspider.com/Chemical-Structure.21782117.html?rid=29d23737-fcc8-4153-a4e8-84a12524310a
- http://www.chemicalize.org/structure/#!mol=C1%5BC%40%40H%5D2%5BC%40H%5D3%5BC%40%40H%5D%28%5BC%40H%5D1%5BC%40H%5D4%5BC%40%40H%5D2O4%29%5BC%40%40%5D5%28C%28%3DC%28%5BC%40%5D3%28C5%28Cl%29Cl%29Cl%29Cl%29Cl%29Cl&source=fp
- http://www.inchem.org/documents/ehc/ehc/ehc130.htm
- http://www.toxipedia.org/display/toxipedia/Endrin
- http://www.popstoolkit.com/about/chemical/endrin.aspx
- http://www.who.int/water_sanitation_health/dwq/chemicals/endrin.pdf
- http://water.epa.gov/drink/contaminants/basicinformation/endrin.cfm
- http://www.nrdc.org/breastmilk/diel.asp
- http://www.atsdr.cdc.gov/ToxProfiles/tp89.pdf
- http://www.epa.gov/iris/subst/0363.htm
- http://www.inchem.org/documents/jmpr/jmpmono/v070pr13.htm
- http://www.faa.gov/data_research/research/med_humanfacs/oamtechreports/1970s/media/AM70-11.pdf
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1022099/pdf/westjmed00088-0044.pdf
- http://whqlibdoc.who.int/ehc/WHO_EHC_130_eng.pdf
 | |
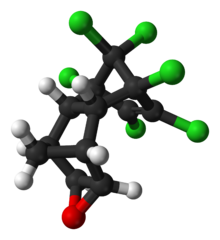 | |
| Names | |
|---|---|
| IUPAC name
(1R,2S,3R,6S,7R,8S,9S,11R)-3,4,5,6,13,13-Hexachloro-10-oxapentacyclo[6.3.1.13,6.02,7.09,11]tridec-4-ene
| |
| Other names
Mendrin, Compound 269, (1aR,2S,2aS,3S,6R,6aR,7R,7aS)-3,4,5,6,9,9-hexachloro-1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-dimethanonaphtho[2,3-b]oxirene, 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-endo,endo-5,8-dimethanonaphthalene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
| UNII | |
| |
| |
| Properties | |
| C12H8Cl6O | |
| Molar mass | 380.907 g/mol |
| Appearance | Colorless to tan crystalline solid |
| Density | 1.77 g/cm3 [1] |
| Melting point | 200 °C (392 °F; 473 K) |
| 0.23 mg/L[2] | |
| Vapor pressure | 2.6 x 10-5 Pa[1] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | noncombustible [3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3 mg/kg (oral, monkey) 16 mg/kg (oral, guinea pig) 10 mg/kg (oral, hamster) 3 mg/kg (oral, rat) 7 mg/kg (oral, rabbit) 1.4 mg/kg (oral, mouse)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.1 mg/m3 [skin][3] |
REL (Recommended)
|
TWA 0.1 mg/m3 [skin][3] |
IDLH (Immediate danger)
|
2 mg/m3[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Endrin is an organochloride with the chemical formula C12H8Cl6O that was primarily used as an insecticide, as well as a rodenticide. It is a colourless odorless solid, although commercial samples are often off-white. Endrin was also sold as an emulsifiable solution known commercially as Shell Endrix. The compound endrin became infamous as a persistent organic pollutant and for this reason it is banned in many countries. [5]
Endrin can be found in our environment mainly in bottom sediments of bodies of water. [6] It is found in the environment as either endrin, endrin aldehyde or endrin ketone. Exposure to endrin can occur via inhalation, ingestion of substances containing endrin or skin contact. [7] Upon entering the bodies of animals and humans, it can be stored in body fats and acts as a neurotoxin on the central nervous system. It can cause convulsions and seizures or even death.[8]
Although endrin is not current classified as a human carcinogen, it is still a toxic chemical. [9] All use of endrin in the United states has been canceled by 1991. Food import restrictions were also put in place for the likelihood that some coutnries may have still been using endrin as a pesticide agent at that time. [7]
Production and Application
[edit]Endrin is produced via a multistep route from hexachlorocyclopentadiene. It begins by condensing vinyl chorlid with hexachlorocyclopentadiene. This product is then dehyrdochlorinated and followed by a reaction with cyclopentadiene. This forms isodrin which is then epoxidized by peracetic or perbenzoic acid to create endrin. [10] Endrin is also known as being a stereoisomer of dieldrin. [11]
Endrin was applied in many different ways. It was formulated into emulsifiable concentrates (ECs), wettable powders (WPs), granules, field strength dusts (FSDs), and pastes. It was then applied via high pressure spraying with a hand gun, spraying of orchard with an air blast, dusting potatoes, spraying row crops or application from an aircraft. [12]
Use
[edit]Endrin was formulated as emulsifiable concentrates (ECs), wettable powders (WPs), granules, field strength dusts (FSDs), and pastes. The product could then be applied by aircraft or by handheld sprayers in its various formulations. [12] Endrin has been used as an agriculture insecticide on tobacco, apple trees, cotton, sugar cane, rice, cereal, and grains. [13] It is effective against a variety of species such as: cotton boll worms, corn borers, cut worms and grass hoppers. [14] It has also been used to control rodents and birds. [15] As a pre-harvest treatment, endrin’s main use worldwide has been its application as a spray on cotton for the control of insects. In Australia, endrin is also used for insect control on tobacco. As a post-harvest treatment, endrin is sprayed onto the ground under the trees to control for rodents during autumn and spring. Its last use is as seed treatment. Cotton seeds in the United States and bean seeds in Australia are treated by endrin. [16] In Malaysia, a solution of endrin was used to rid mine pools and fish ponds of all fish prior to restocking.[17]
History
[edit]Endrin was first developed in 1950 by J. Hyman & Company and was used globally until the early 1970s. It was licensed to be manufactured by Shell International and Velsicol chemical companies. Production began in the United States by Shell and Velsicol and in the Netherlands by Shell. Due to its toxicity, it has been banned or severely restricted in many countries. In 1982, Shell discontinued the manufacturing of endrin.[10]
It was estimated that 2.3-4.5 million kilograms of endrin were sold in the USA in 1962. In 1970, Japan imported 72,000 kilograms of endrin. From 1963, Bali used 171 to 10,700 kilograms of endrin annually for the production of rice paddies until use of the compound was discontinued in 1972.[10] Taiwan reported to show higher levels of organochlorine pesticides including endrin, compared to other Asian countries such as Thailand and Vietnam. Over two million kilograms of organochlorine pesticides was estimated to be released into the environment annually in 1950s-1970s. Taiwan banned the use of endrin in 1989.
The Stockholm Convention on persistent organic pollutants that came in effect May 2004 listed endrin as one of the 12 initial persistent organic pollutants (POPs) that have been causing adverse effects on humans as well as the ecosystem. The convention required the participating parties to take measures to eliminate or restrict the production of POPs
Health Effects
[edit]Endrin is toxic with an LD50 of 3 mg/kg (oral, rat).[18] Acute endrin poisoning in humans affects primarily the nervous system. Food contaminated with endrin caused several clusters of poisonings worldwide, especially affecting children.[5] Orally ingested endrin is eliminated mostly in feces.[19][full citation needed] It is very toxic to aquatic organisms, namely fish, aquatic invertebrates, and phytoplankton. The U.S. EPA has set a freshwater acute criterion of 0.086 ug/L and a chronic criterion of 0.036 ug/L. In saltwater, the numbers are acute 0.037 and chronic 0.0023 ug/L.[20][full citation needed] Human health contaminate criterion for water plus organism is 0.059 ug/L.[21][full citation needed] Drinking water limits (maximum contaminant level (mcl)) is set to 2 ppb.[22][full citation needed]
Endrin is considered not classifiable as to carcinogenicity to humans by the United States National Toxicology Program (USNTP). The cyclodiene pesticides like chlordane, aldrin, dieldrin, and endrin are found to have much higher mammalian toxicity than other pesticides like DDT.
Environmental Issues
[edit]Starting in 1970s, the use of insecticides like dieldrin and endrin has been prohibited due to their high toxicity and long persistence in the environment.[23] A definitive detection of the residues was not possible until 1971 when mass spectrometer started being used as detector in gas chromatography.[24] As of 2004, the use of endrin is banned in countries that ratified the Stockholm Convention.[5] However, the detection of these chemicals in the environment has been reported across the world up to 2005,[25] even though the frequency is low due to its relatively small-scale use and very low concentrations. (Chlorinated Pesticides, pg. 54)
Endrin, including other pesticides, enter the environment through direct treatment or as they are washed off from crops during rainfall. These were found in water, sediments, atmospheric air and biotic environment, even after their uses have been stopped.[26] Common properties of organochlorine pesticides include strong resistance to degradation, low solubility in water but high solubility in lipids. (pg. 49) The lipophilic tendency leads to bioaccumulation in fatty tissues of organisms, mainly those dwelling in water. A significant bioconcentration factor of 1335-10,000 has been reported in fish. (ref: factsheet) Endrin binds very strongly to organic matter in soil and aquatic sediments due to their high adsorption coefficient, [27] making it less likely to leach into groundwater, even though contaminated groundwater samples have been found. (ref: factsheet) EPA in 2009 released data indicating that the endrin in soil could last up to 14 years or more.[28]
Removal from the Environment
[edit]Before endrin's toxicity was known, disposal methods consisted of land disposal. Due to endrin's toxicity, more precuatious measure have been taken. Two possible methods are chemical treatment (reductive dechlorination) or incineration. These disposal methods are applied to endrin and endrin aldehyde.[7] Ketone endrins can be removed from the environment via photodecomposition, forming δ-ketoendrin, and microbial degradation. Microbial degradation depends on species in the soil as well as anaerobic conditions. Biodegradation is then aided by fungi and bacteria to form the major end product, delta-ketoendrin.[12] Even though endrin binds very strongly to soil, possibility of using phytoremediation has been proposed using crops in Cucurbitaceae family.
- ^ a b "Endrin". Deutsche Gesellschaft für Technische Zusammenarbeit. Retrieved 13 March 2015.
- ^ "Endrin (PDS)". IPCS. Retrieved 13 March 2015.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0252". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Endrin". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. Retrieved 19 March 2015.
- ^ a b c The National Poison Centre (October 12, 1998). "Poison Control: Dangers of 'persistent organic pollutants' in the environment". The New Straits Times Press (Malaysia) Berhad. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Persistent Organic Pollutants Toolkit". Endrin. Hatfield Consultants. 1995. Retrieved March 14, 2015.
- ^ a b c U.S. Department of Health and Human Services (August 1996). "Toxilogical Profile for endrin" (PDF). Agency for Toxic Substances and Disease Registry.
- ^ Revzin, Alvin (July 1970). "Some Acute and Chronic Effects of Endrin on the Brain" (PDF). Federal Aviation Administration. Retrieved March 14, 2015.
- ^ "Integrated Risk Information System". U.S. Environmental Protection Agency. September 1988. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b c van Esch, G. T.; van Heemstra-Lequin, E. A. H. (1992). "Environmental Health Criteria 130: Endrin". International Programme on Chemical Safety. World Health Organization. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Organochlorine Pesticides Overview". Center for Disease Control and Prevention. December 2013. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b c "Endrin Health and Safety Guide". International Programme on Chemical Safety. World Health Organization. 1991. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Basic Information about Endrin in Drinking Water". EPA. United States Environmental Protection Agency. February 9, 2014. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ World Health Organization (January 1975). "Data Sheets on Pesticides No. 1". IPCS InChem. Retrieved March 2015.
{{cite journal}}: Check date values in:|access-date=(help) - ^ "Healthy Milk, Healthy Baby - Chemical Pollution and a Mother's Milk". NRDC. National Resources Defense Council. March 25, 2005. Retrieved March 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ FAO and WHO (December 1, 1970). "1970 Evaluations of somePesticide Residues in Food". The Monographs. Retrieved March 2015.
{{cite journal}}: Check date values in:|access-date=(help) - ^ "Integrated Management Techniques to Control Nonnative Fishes" (PDF). Upper Midwest Environmental Sciences Center. United States Geological Survey. December 2003.
- ^ Endrin in the ChemIDplus database
- ^ WHO report: 1970 Evaluations of some pesticide residues in food. 1970
- ^ US EPA Criteria for Aquatic Life (pdf)
- ^ US EPA human health criteria document
- ^ US EPA Drinking water document
- ^ "Bioremediation of the organochlorine pesticides, dieldrin and endrin, and their occurrence in the environment".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Zitko, Vladimir. Chlorinated Pesticides: Aldrin, DDT, Endrin, Dieldrin, Mirex.
- ^ "Bioremediation of the organochlorine pesticides, dieldrin and endrin, and their occurrence in the environment".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Chopra, A. K.; Sharma, Mukesh K. "Bioaccumulation of organochlorine pesticides in aquatic system-an overview".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Zitko, Vladimir. Chlorinated Pesticides. p. 53.
- ^ "Technical Factsheet on: Endrin" (PDF). EPA. ?. Retrieved 4 March 2013.
{{cite web}}: Check date values in:|date=(help)

