User:Hkang10/sandbox
Noyori asymmetric hydrogenation
[edit]Introduction
[edit]The Noyori asymmetric hydrogenation of ketones is a chemical reaction which adds hydrogen across one face of ketone. This reaction is catalyzed using chiral ruthenium complex catalysts introduced by Ryoji Noyori since 1987. [1] He shared half of the Nobel Prize in Chemistry in 2001 with William S. Knowles for the study of the asymmetric hydrogenation. Noyori Assymetric hydrogenation has an excellent stereoselectivity and very wide scope of reactions depends on the catalyst. BINAP-Ru catalyst is used for the asymmetric hydrogenation of functionalized ketones[2] and BINAP/diamine-Ru catalyst is used for the asymmetric hydrogenation of simple ketones.[3]

Due to its high stereoselectivity, the BINAP-Ru catalytic system is widely used in the pharmaceutical industry. Drugs such as an antibacterial levofloxin, an antibiotic carbapenem, and an antipsychotic agnet BMS181100 are synthesized industrially using Noyori asymmetric hydrogenation.[4]
History
[edit]The history of asymmetric ketone hydrogenation mostly relied on metal-hydride chemistry largely developed by H.C Brown. Chemoselective hydrogenation of ketone was acheived by using the stoichiometric NaBH4, through the coordination of boron to the carbonyl oxygen.[5]. The diastereoselective ketone hydrogenation was acheived by using Selectride.[6] Enantioselective ketone hydrogenation was acheived by chiral stoichimetric reagent[7] or by the Corey-Bakshi-Shibata(CBS) method. However, until recently catalytic asymmetric hydrogenation of ketone was not acheived.
In 1991, Noyori and coworkers modified BINAP-Ru dicarboxylate, which was used for asymmetric hydrogenation of olefin, to the BINAP-Ru dihalide catalyst. The new catalyst could catalyze the asymmetric hydrogenation of various functionalized ketones.[8]. While the BINAP-Ru dicarboxylate could only efficiently catalyze the hydrogenation of olefins, the BINAP-Ru dihalide could catalyze both the hydrogenation of olefins and the hydrogenation of functionalized C=O bond[9].

Even though the BINAP-Ru dihalide catalyst could reduce functionalized ketones, the hydrogenation of simple ketones has remaind as an challenge. In 1995, Noyori discovered that the RuCl2 (diphosphane)2 (diamine)2 complex can catalyze the hydrogenation of simple ketones[10]. This system also had chemoselectivity on C=O bond over the C=C bond[11]. The diastereoselectivity[12] and the enantioselectivity[3] could be achieved at the same time using chiral BINAP ligand.

Mechanism and Selectivity
[edit]BINAP-Ru
[edit](A) The BINAP-Ru dihalide precatalyst gets hydride from H2 and forms Ru-monohydride intermediate while giving off HCl.[13] (B) The ruthenium center of the catalyst coordinates to the oxygen atoms in the ester compound. Because of the chirality of the BINAP ligand, one of the two possible diasteremeric transition states is favored (The transition state on the left is favored over the other because of the large R1/ Ph steric hindrance). (C) Ester gets proton, and hydride transfers from the catalyst to the carbonyl carbon. (D) Hydrogenated ester compound leaves the catalyst and solvent coordinate back to the catalyst. The (R)-BINAP-Ru catalyze the synthesis the (S)-Product, and the (S)-BINAP Ru catalyze the synthesis the (R)-product with high ee.[14] (E) Again, the dehydrated BINAP-Ru catalyst is utilized by the addition of another hydride from H2. The newly activated Ru-monohydride re-participates in the catalytic cycle.
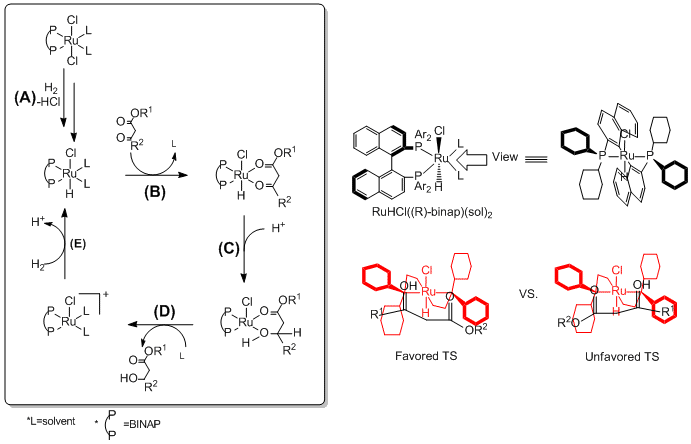
BINAP/diamine-Ru
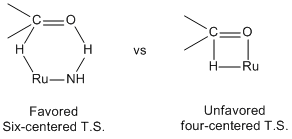
[edit]In BINAP-Ru catalytic system, the hydrogenation of functionalized ketone is catalyzed by a four-center transition state(Transition state model on the right) which forms a metal alkoxide intermediate. Unlike the BINAP-Ru, the BINAP/diamine-Ru catalytic system forms the six membered pericyclic transition state(Transition state model on the left) which directly leads to the product. This non-classical metal-ligand bifunctional transition state facilitates the hydrogenation of C=O bond with higher rate and the higher chemoselectivity.[11]
The RuCl2 (diphosphane)2 (diamine)2 catalyst can hydrogenate simple cyclic ketones diastereoselectively.[12] In the presence of base, cyclic ketones are deprotonated and racemized. In the transition state, the ruthenium monohydride moiety acts as a bulky group (marked red on the scheme below). The product is predictable in the way that the catalyst approaches from the less hindered side.(see also Dynamic kinetic resolution)

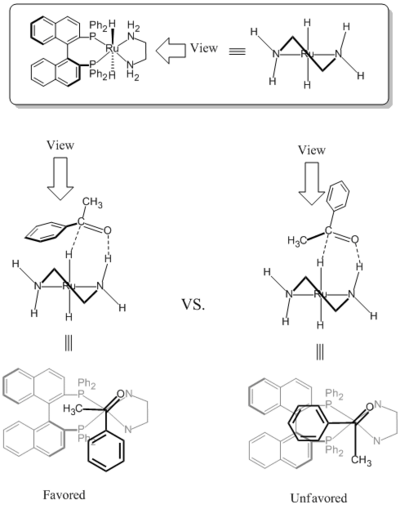
The chirality of the diamine ligand made it possible for the BINAP/diamine-Ru complex possible to reduce simple ketones enantioselectively. Due to the steric hindrance between the BINAP ligand and the large substituent group on ketone (phenyl ring on the scheme below), the less steric hindered transition state is favored as expected.[3] The simple ketones include aromatic, heteroaromatic, and alkenyl ketones.
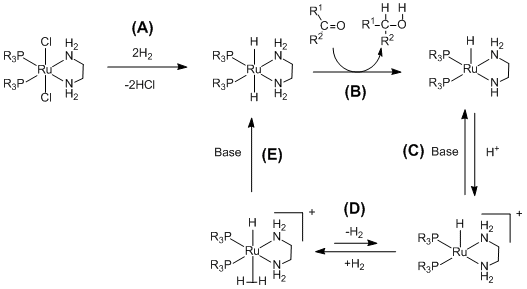
(A) The ruthenium (diphosphane)2 (diamine)2 complex is activated by the addition of the hydrogen gas. (B) The activated catalyst transfers hydrogen and hydride chemoselectively to the ketone through a pericyclic transition state.[11](C)(D)(E)The ruthenium complex can then react with hydrogen to reform the ruthenium dihydride with the assistance of base.
Scope
[edit]BINAP-Ru
[edit]Further developed from previously introduced BINAP-Ru dicarboxylate catalyst, the BINAP-Ru dihalide catalyst can catalyze the asymmetric hydrogenation of various α-,β- functionalized ketones.[8] Eventhough, it can catalyze the hydrogenation of some ketones, the scope is limited to the functionalized ketones which have a nearby N,O or X (halide) coordinating group.

BINAP/diamine-Ru
[edit]The BINAP-Ru dihalide can only catalyze the asymmetric hydrogenation of functionalized ketone. But the BINAP/diamine-Ru catalyst can catalyze both functionalized ketone and simple ketones, which does not have a functional group to coordinate. The RuCl2(diamine)2(diphosphane)2 caltayst can catalyze cyclic ketones diastereoselectively[12], and BINAP/diamine-Ru catalyst can catalyze aromatic, heteroaromatic, and olefinic ketones enantioselectively.[3] Better stereoselectivity is acheived when one substituent is larger than the other substituent. When both substituents in ketone are large or both substituents are about the similar size, the stereoselectivity is not guaranteed.

Application
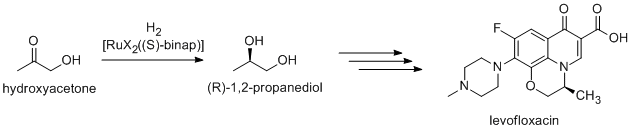
[edit]An antibacterial levofloxacin is synthesized using (R)-1,2-propandiol, which is synthesized from hydroxyacteone using Noyori asymmetric hydrogenation(Takasago Co./Daiichi Pharmaceutical Co.).[4]
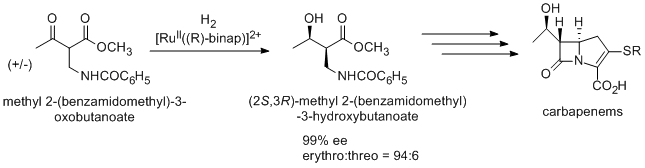
An antibiotic carbapenem is also synthesized using Noyori asymmetric hydrogenation. In the synthesis, (2S,3R)-methyl 2-(benzamidomethyl)-3-hydroxybutanoate is synthesized from racemic methyl 2-(benzamidomethyl)-3-oxobutanoate by the dynamic kinetic resolution. After several steps, carbapenem is synthesized(Takasago International Co.).[4]
An antipsychotic agent BMS 181100 is synthesized using BINAP/diamine-Ru catalyst. Enantioselectivity is achieved by steric hindrance between the chiral BINAP ligand and the aromatic group on the reactant.[4]

Reference
[edit]- ^ Noyori, R.; Ohkuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. (1987), "Asymmetric hydrogenation of .beta.-keto carboxylic esters. A practical, purely chemical access to .beta.-hydroxy esters in high enantiomeric purity", Journal of the American Chemical Society, 109 (19): 5856–5858, doi:10.1021/ja00253a051
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Mashima, K.; Kusano, K.-h.; Sato, N.; Matsumura, Y.-i.; Nozaki, K.; Kumobayashi, H.; Sayo, N.; Hori, Y.; Ishizaki, T. (1994), "Cationic BINAP-Ru(II) Halide Complexes: Highly Efficient Catalysts for Stereoselective Asymmetric Hydrogenation of .alpha.- and .beta.-Functionalized Ketones", The Journal of Organic Chemistry, 59 (11): 3064–3076, doi:10.1021/jo00090a026
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Noyori, R.; Ohkuma, T. (2001), "Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones", Angewandte Chemie International Edition, 40 (1): 40–73, doi:10.1002/1521-3773(20010105)40:1<40::aid-anie40>3.0.co;2-5, PMID 11169691
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Noyori, R. (2002), "Asymmetric Catalysis: Science and Opportunities (Nobel Lecture)", Angewandte Chemie International Edition, 41 (12): 2008–22, doi:10.1002/1521-3773(20020617)41:12<2008::aid-anie2008>3.0.co;2-4, PMID 19746595
- ^ Schlesinger, H. I.; Brown, H. C.; Hoekstra, H. R.; Rapp, L. R. (1953), "Reactions of Diborane with Alkali Metal Hydrides and Their Addition Compounds. New Syntheses of Borohydrides. Sodium and Potassium Borohydrides1", Journal of the American Chemical Society]], 75: 199–204, doi:10.1021/ja01097a053
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Brown, H. C.; Krishnamurthy, S. (1972), "Lithium tri-sec-butylborohydride. New reagent for the reduction of cyclic and bicyclic ketones with super stereoselectivity. Remarkably simple and practical procedure for the conversion of ketones to alcohols in exceptionally high stereochemical purity", Journal of the American Chemical Society, 94 (20): 7159–7161, doi:10.1021/ja00775a053
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Ramachandran, P. V.;,Brown, H. C. (1996), "Recent Advances in Asymmetric Reductions with B-Chlorodiisopinocampheylborane", Journal of the American Chemical Society, ACS Symposium Series, 641: 84–97, doi:10.1021/bk-1996-0641.ch005, ISBN 0-8412-3381-0
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Mashima, K.; Kusano, K.-h.; Sato, N.; Matsumura, Y.-i.; Nozaki, K.; Kumobayashi, H.; Sayo, N.; Hori, Y.; Ishizaki, T. (1994), "Cationic BINAP-Ru(II) Halide Complexes: Highly Efficient Catalysts for Stereoselective Asymmetric Hydrogenation of .alpha.- and .beta.-Functionalized Ketones", The Journal of Organic Chemistry, 59 (11): 3064–3076, doi:10.1021/jo00090a026
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Kitamura, M.; Ohkuma, T.; Inoue, S.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Ohta, T.; Takaya, H.; Noyori, R. (1988), "Homogeneous asymmetric hydrogenation of functionalized ketones", Journal of the American Chemical Society, 110 (2): 629–631, doi:10.1021/ja00210a070
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Ohkuma, T.; Ooka, H.; Hashiguchi, S.; Ikariya, T.; Noyori, R. (1995), "Practical Enantioselective Hydrogenation of Aromatic Ketones", Journal of the American Chemical Society, 117 (9): 2675–2676, doi:10.1021/ja00114a043
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c Ohkuma, T.; Ooka, H.; Ikariya, T.; Noyori, R. (1995), "Preferential hydrogenation of aldehydes and ketones", Journal of the American Chemical Society, 117 (41): 10417–10418, doi:10.1021/ja00146a041
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c Ohkuma, T.; Ooka, H.; Yamakawa, M.; Ikariya, T.; Noyori, R. (1996), "Stereoselective Hydrogenation of Simple Ketones Catalyzed by Ruthenium(II) Complexes", The Journal of Organic Chemistry, 61 (15): 4872–4873, doi:10.1021/jo960997h
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Kitamura, M.; Tokunaga, M.; Noyori, R. (1993), "Quantitative expression of dynamic kinetic resolution of chirally labile enantiomers: stereoselective hydrogenation of 2-substituted 3-oxo carboxylic esters catalyzed by BINAP-ruthenium(II) complexes", Journal of the American Chemical Society, 115: 144–152, doi:10.1021/ja00054a020
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Noyori, R.; Ohkuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. (1987), "Asymmetric hydrogenation of .beta.-keto carboxylic esters. A practical, purely chemical access to .beta.-hydroxy esters in high enantiomeric purity", Journal of the American Chemical Society, 109 (19): 5856–5858, doi:10.1021/ja00253a051
{{citation}}: CS1 maint: multiple names: authors list (link)
